
 Audited Supplier
Audited Supplier
In This Store
Category:Fluid Equipment > Ultrafiltration
Product Name:Ultrafiltration system / tangential flow filtration/LDS-L2TANGENTIAL FLOW FILTRATION
Price(USD):Negotiable
Company:Suzhou Aitesen Pharmaceutical Equipment Co., Ltd.
Factory Location: Suzhou, Jiangsu province
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Sample Provided: no
Payment Terms: other
TANGENTIAL FLOW FILTRATION
BRIEF INTRODUCTION
AITESEN LDS series Tangential Flow Filtration is mainly applied in the research of tangential flow ultrafiltration technology for micro & nano drugs and biological products, providing excellent technical services and solutions.
The equipment can be used for process research experiments on small-scale samples, as well as for large-scale process validation experiments. The equipment is equipped with a touch screen operating system and software programs for automatic data recording, supporting data tracing and export. The process is reliable and suitable for programmatic amplification. The components comply with pharmaceutical industry standards, they are safe and reliable.
TFF:
Tangential Flow Filtration (TFF) is currently a widely used membrane separation technology. The sample is driven by pressure, and the content is separated by membrane filtration according to different molecular sizes, achieving the goals of removing heat, replacing the solution environment, purification ,and concentration.
PRINCIPLE:
The implementation process of TFF is fundamentally different from dead end filtration. As the filtrate passes through the membrane components, the sample solution continuously passes through the membrane surface in a vertical direction.
MEMBRANE PARTS:
用于切向流过滤的膜组件通常是膜组件或中空纤维柱,两者都有多种型号,以满足不同的使用需求。膜材料的种类和孔径、溶液循环流速、膜两侧压力、样品温度、膜两侧工艺、样品温度、工艺时间等参数对于切向流过滤非常重要。他们决定了最终的工艺效果,以及大规模生产的可行性和具体计划。实验室规模的切向流过滤设备致力于获得可行的工艺方案和可靠的工艺参数,确定适合大规模实验和生产的工艺参数,为设备或系统选型提供有效依据。
T FF流程图
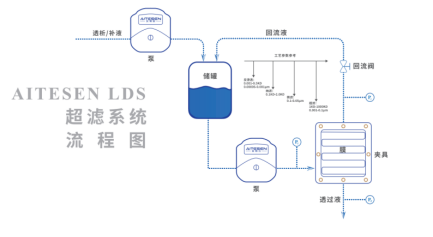
应用领域
核酸药物和疫苗
mRNA-LNP、siRNA、聚合物纳米颗粒等
化疗脂质体
盐酸阿霉素脂质体
盐酸伊立替康脂质体
柔红霉素脂质体
复合脂质体等
解决方案系统位移
替代缓冲溶液
纳米药物溶液的外置水相置换
细胞产物分离纯化
发酵液细胞的收集和纯化
澄清过程
质粒、单克隆抗体、蛋白纯化
质粒、mRNA、siRNA等
(二)LDS-L2TANGENTIAL FLOW FILTRATION
TECHNICAL PARAMETER:
|
Model |
LDS-L2 |
|
Minimum Sample Volume |
≤30mL |
|
Pressure Range |
-1-5bar(The system is equipped with a pressure sensor) |
|
Maximum Flow Rate |
≥2200mL/min |
|
Accuracy |
±0.5% |
|
Weight Range |
0–4100g |
|
Control Parameters |
Pf (feed pressure), Pr (reflux pressure), Pp (permeation pressure), TMP (transmembrane pressure), △ P (pressure drop), etc. |
|
Operation Mode |
Fully automatic implementation of TFF process, which can execute complex TFF processes through integrated monitoring of user specified settings within the integrated PLC software platform.
|
|
Software |
Special software for automated TFF system, with simple operating logic, real-time tracking, real-time data analysis, and compliance with FDA 21 CFR Part 11 data audit tracking. |
|
Software Function Description |
Three levels of password permissions: operator, technician, administrator, and functional operations are divided according to different permissions; Process parameters such as flow rate, flow rate, and pressure can be monitored during the process; Process parameters such as flow rate, flow rate, and pressure can be monitored during the process; When the pressure at the sampling or filtering end reaches or exceeds the preset value, the system will trigger an alarm and/or shutdown function; Key parameters in the process (such as sample volume, flow rate, flow rate, pressure, and time of each process segment) can be recorded and exported. Data display, recording, and export can be in the form of trend charts, tables, and batch record reportssimultaneously. During equipment operation, the main interface of the operation screen can display real-time dynamic changes in sample volume, current sample flow direction, and the operating status of various components. |
|
Applicable Membrane Materials |
PES/cellulose membrane package, hollow fiber |
|
Film Package Specification |
5~1000kD;0.01~0.1m2 |
EQUIPMENT FEATURES:
Automated Ultrafiltration System
It can achieve unmanned automated TFF process and execute complex TFF processes through integrated monitoring of user specified settings within the integrated PLC software platform.
Professional Operating System
Special software with simple operating logic, real-time tracking, real-time data analysis, and compliance with FDA 21 CFR PART 11 data audit tracking.
Drug R&D Orientation
The equipment design is based on the process requirements and regulatory specifications for drug development and production, and can be used for the implementation of processes such as purification, solvent removal, and concentration of liposome, lipid nanoparticles, and other formulations.

Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


