
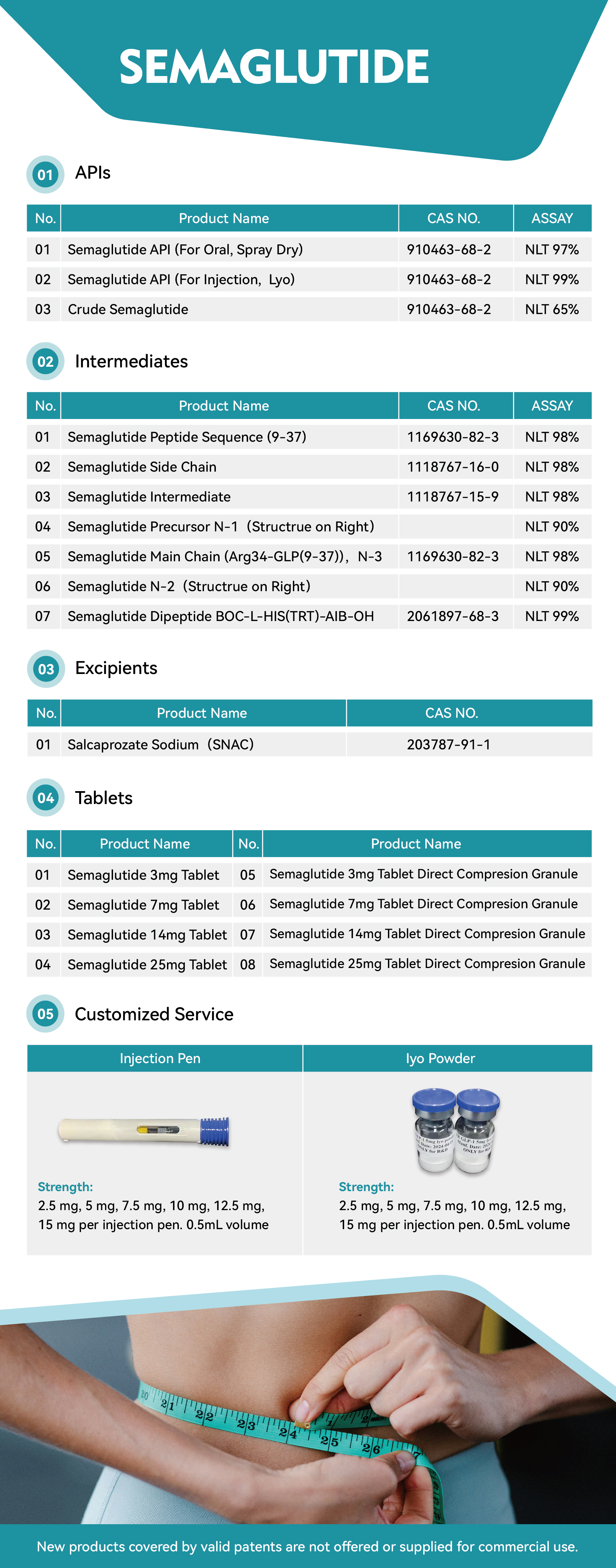
 Ten years of qualification
Ten years of qualification
 Audited Supplier
Audited Supplier
In This Store
Category:Active Pharmaceutical Ingredients > Digestive System Drugs
Product Name:Semaglutide
CAS No.:910463-68-2
Standard:USP, BP, EP
Price(USD):1~11
Company:Arshine Group Co.,Ltd.
Grade: Pharmaceutical Grade
Factory Location: lock 14, No.100, Luyun Road, Changsha 410205, Hunan, China.
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Sample Provided: no
Payment Terms: L/C
Semaglutide is a medication belonging to the class of glucagon-like peptide-1 receptor agonists (GLP-1 agonists). It is primarily used in the management of type 2 diabetes mellitus to help improve blood sugar control and promote weight loss.
Semaglutide is administered as a subcutaneous injection and is prescribed by healthcare providers as an adjunct to diet and exercise for individuals with type 2 diabetes who have not achieved adequate glycemic control with other antidiabetic medications.
Key uses and benefits of Semaglutide:
1:Blood Sugar Control: Semaglutide works by mimicking the actions of incretin hormones, particularly GLP-1. It increases insulin secretion in response to elevated blood sugar levels and reduces the production of glucagon, leading to lower blood glucose levels after meals.
2:Weight Loss: In addition to its antidiabetic effects, Semaglutide has been found to promote weight loss in individuals with type 2 diabetes. This can be beneficial for overweight or obese patients who are struggling to manage their diabetes and weight simultaneously.
3:Cardiovascular Benefits: Some clinical studies have suggested that Semaglutide may have cardiovascular benefits by reducing the risk of major adverse cardiovascular events in certain individuals with type 2 diabetes and established cardiovascular disease.
|
Product Name |
Semaglutide |
CAS No. |
910463-68-2 |
||
|
Molecular Formula |
C₁₉H₂₁N₄₅O₅ |
Molecular Weight |
4113.58 |
||
|
Sequence |
His-Aib-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-GIn-Ala-Ala -N6-[N- (17-carboxy-1-oxoheptadecyl-L- γ-glutamyl[2- (2-aminocthoxy)ethoxy]acetyl (2-2-aminocth oxy)cthoxy]acetyll-Lys-Glu-Phe-Ile-Ala-Trp-Lcu-Val-Arg-Gly-Arg- Gly-OH |
||||
|
Batch No |
SMT-230312 |
Quantity |
16.4g |
||
|
Source |
Synthetic |
Mfgr.Date |
2023-03-12 |
||
|
Test.Date |
2023-03-18 |
Retest Date |
2026-03-11 |
||
|
Tests |
Acceptance Criteria |
Results |
|||
|
Appearance |
White or off-white powder |
White powder |
|||
|
Solubility |
Freely soluble in water |
Conforms |
|||
|
Specific optical rotation[ul?。 |
15.0° to-10.0 °(anhydrous and sodium ion-free substance C=10mg/ml,H₂O) |
10.3° |
|||
|
Identification by HPLC |
The retention time of the principal peak of the test solution corresponds to that of the reference solution, as obtained in the Assay. |
Conforms |
|||
|
Molecular Weight |
4113.58±1.0 |
Conforms |
|||
|
pH |
7.0-9.0 |
7.9 |
|||
|
Water Content |
NMT 8.0% |
4.9% |
|||
|
Purity(HPIC) |
NLT 95.0% |
99.0% |
|||
|
Related substances(HPIC) |
The largest individual impurity test NMT 3.0% |
0.4% |
|||
|
The total impurities test NMT 5.0% |
1.0% |
||||
|
Residual solvent |
DCM |
NMT 6000ppm |
N.D |
||
|
ACN |
NMT 410ppm |
8ppm |
|||
|
MetOH |
NMT 3000ppm |
N.D |
|||
|
DMF |
NMT 880ppm |
N.D |
|||
|
Isopropyl ether |
NMT 5000ppm |
N.D |
|||
|
Isopropanl |
NMT 5000ppm |
N.D |
|||
|
Amino Acid analysis |
Asp |
0.8~1.2 |
0.92 |
||
|
His |
0.8~1.2 |
1.10 |
|||
|
Tyr |
0.8~1.2 |
0.95 |
|||
|
Lys |
0.8~1.2 |
0.98 |
|||
|
Amino Acid analysis |
lle |
0.8~1.2 |
1.11 |
|
Leu |
1.6~2.4 |
2.23 |
|
|
Val |
.6~2.4 |
2.12 |
|
|
Arg |
.6~2.4 |
.98 |
|
|
Thr |
.6~2.4 |
1.72 |
|
|
Phe |
1.6~2.4 |
2.25 |
|
|
Ser |
2.4~3.6 |
2.56 |
|
|
Ala |
2.4~3.6 |
2.89 |
|
|
Gly |
3.2~4.8 |
3.56 |
|
|
Glu |
4.0-6.0 |
4.92 |
|
|
Aib |
N/A |
N/A |
|
|
AEEA |
N/A |
N/A |
|
|
Acid group ions |
TFA |
NMT 0.1% |
N.D |
|
PO4³* |
NMT<0.1% |
N.D |
|
|
Cl≤ |
NMT 0.1% |
0.003% |
|
|
HAC< |
NMT 0.1% |
N.D |
|
|
Microbiological tests |
Total microbacterial count |
<1000CFU/g |
Conforms |
|
Yeast and Mould |
≤100 CFU/g |
Conforms |
|
|
Escherichia Coil presence |
Negative |
Conforms |
|
|
Salmonella |
Negative |
Conforms |
|
|
P.Aeruginosa |
Negative |
Conforms |
|
|
S.Aureus |
Negative |
Conforms |
|
|
Total heavy metal test |
NMT 10.0 ppm |
Conforms |
|
|
Sodium ion |
NMT 5.0% |
2.2% |
|
|
High molecular protein |
NMT 0.5% |
0.03% |
|
|
Peptide Content |
NLT 80.0% |
97.5% |
|
|
Conclusion: conformed with the product standards. |
|||

Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


