
 One year of qualification
One year of qualification
 Audited Supplier
Audited Supplier
In This Store
Category:Preparation Equipment > Isolator
Product Name:Marya Customized CE-Certificated Linkage Production Line Isolator Manufacturer
Price(USD):US$14000
Company:Marya pharma engineering Co.,Ltd
Factory Location: Changsha,Hunan
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Packaging Information: Wooden case for sea shipment
Sample Provided: no
Payment Terms: L/C
Marya Customized CE-Certificated Linkage Production Line Isolator Manufacturer
Introduction
The linkage production line isolator system is integrally installed on the sterile filling machine or other equipment to form a controlled enclosure or barrier that completely isolates operators and products. By equipping this system for sterile filling, the risk of drug contamination will be effectively reduced and the quality of drugs be greatly improved.
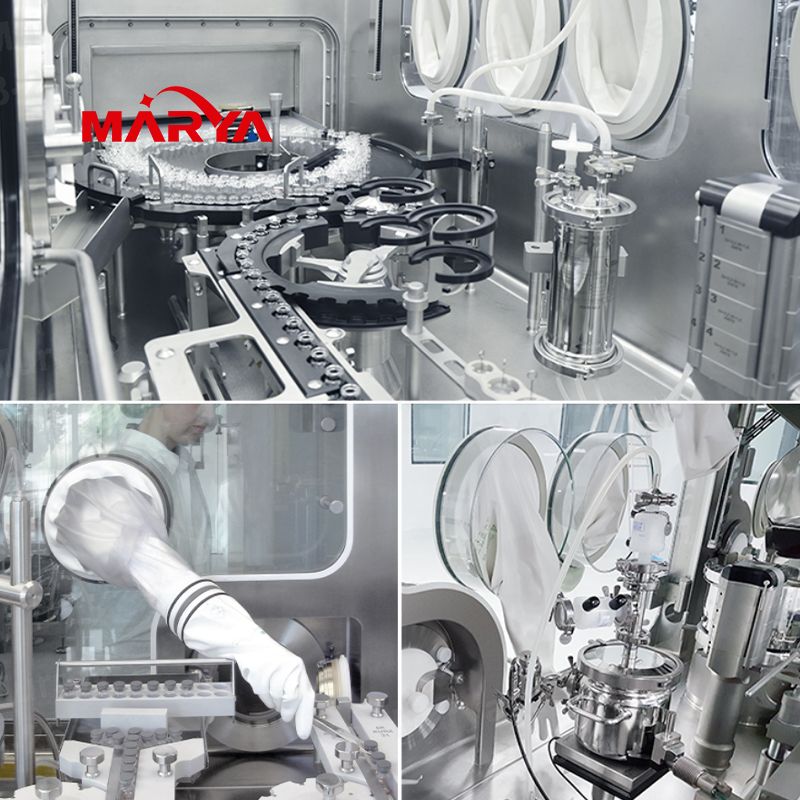
Features
1.Comprehensive product portfolio: isolation systems for water injection, powder injection, prefilled syringe and sterile powder production lines.
2.Stable and reliable environment: reliable and stable class A environment, which meets cGMP, FDA, ISO, ISPE, and pharmacopoeia requirements.
3.New VHPS technology: the latest H2O2 sterilization system, which can achieve rapid sterilization, control the concentration and saturation of H2O2, with good sterilization reproducibility.
4.Intelligent control system: Siemens PLC+IPC control system; intelligent alarms of pressure, H2O2 solution concentration, temperature and humidity, air speed and other monitoring parameters; the system has multi-level access control; comply with FDA 21 CFR part 11 requirements.
5.Independent temperature and humidity control mode: with the integrated independent air conditioning system, it can realize that the internal temperature and humidity in the chamber is not affected by the external environment.
6.MOCK-UP: complete wood model verification service, ergonomic operation qualification and 3D model display service in the middle of the project.
7.Sterilization process development support: qualification and validation services for sterilization cycle development of the whole process can be provided.
8.Customization: support customized isolation system design, and provide systematic closed isolation solutions and CE certification services.
9.Integrated monitoring system: integrated monitoring systems of temperature and humidity, differential pressure, air speed, H2O2 concentration, optical grating, particles and airborne viable bacteria, etc.
10.Multiple sterile transfer solutions: multiple and combined sterile transfer solutions are available to meet customers' diversified production needs.
11.Complete life cycle support: covering consulting, project design, installation scheme design and verification design, etc.
12.Wireless glove leakage detector: multi-head wired glove leakage detector or wireless glove leakage detector is optional.
13.CDCV service: sterilization cycle development, validation studies and services.
Product Parameters
| Name | Parameters |
| Material | 316L |
| Polishing grade | 0.4μm~0.6μm |
| Protection level | OEB 5 (OEL<1ug/m³) |
| Internal arc angel | R≥20 |
| Cleaniness level | Class A |
| Power supply | 380V 50Hz (Note: Other voltages require a transformer) |

Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


