
 Twenty years of qualification
Twenty years of qualification
 Audited Supplier
Audited Supplier
In This Store
Category:Active Pharmaceutical Ingredients > Other Active Pharmaceutical Ingredients
Product Name:99% up Enoxaparin Sodium 679809-58-6
CAS No.:679809-58-6
Standard:USP, BP, EP, JP, In-house Standards
Price(USD):Negotiable
Company:Sinoway Industrial Co. Ltd
Grade: Pharmaceutical Grade
Factory Location: Xiamen, Fujian
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East
Monthly Production Capacity: 1000kg
Packaging Information: 25kg/drum 1kg/bottle
Delivery Lead Time: 7 days after payment
Sample Provided: yes
Payment Terms: L/L
|
Product name |
Enoxaparin Sodium |
|
CAS No. |
679809-58-6 |
|
Molecular Formula |
(C12H16NS2Na3)n |
|
Molecular Weight |
288.428 |
|
Molecular Structure |
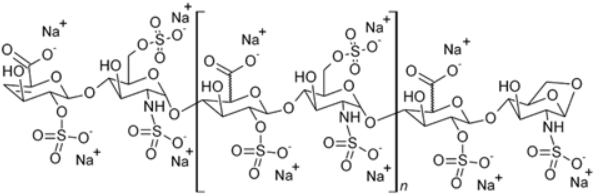 |
|
Quality Standard |
99% up by HPLC |
|
Appearance |
White or almost white powder |
|
COA |
|
ANALYSIS |
SPECIFICATION |
RESULTS |
|
Appearance |
White or almost white powder, |
White powder |
|
Solubility |
Hygroscopic. Solubility: Freely soluble in water |
Complies |
|
Identification |
A: 13C-NMR: the spectrum should be similar with that of standard. Ethanol peak may exist at 18.7ppm±0.5ppm, benzaledyde peak may exist at 131.6ppm±0.5ppm and130.9ppm±0.5ppm in spectrum obtained from test sample. |
The spectrum of test solution is similar to that of enoxaparin sodium RS, Ethanol peak exist at 18.7ppm±0.5ppm. No benzaldehyde peak exist at 131.6ppm±0.5ppm and 130.9ppm±0.5ppm. |
|
B: 1,6 anhydro derivative: 15% to 25% |
20.3% |
|
|
C: The mass-average relative molecular mass ranges between 3800 and 5000 Da. The mass percentage of chains lower than 2000 Da. ranges bwtween 12.0% and 20% The mass percentage of chains between 2000 and 8000 ranges between 68.0% and 82.0% |
The mass-average relative molecular mass : 4384 The mass percentage of chains lower than 2000 Da.: 18.9% The mass percentage of chains between 2000 and 8000 Da.: 70.7% |
|
|
D: Anti-factor Xa/Anti-factor IIa: 3.5 to 5.3 |
3.4 |
|
|
E: Sodium: It complies with the test of sodium |
Complies |
|
|
Appearance of solution |
The solution is clear and not more intensely colored than reference solution |
Complies |
|
Specific absorbance |
The specific absorbance determined at 231 nm ranges between 14.0 and 20.0(dried substance) |
17.3 |
|
Bacterial endotoxins |
< 0.01 IU/Anti-FXa U activity |
< 0.01 IU/Anti-FXa U activity |
|
pH |
6.2 to 7.7 |
7.1 |
|
Loss on drying |
≤10.0% |
1.4% |
|
Nitrogen |
1.5% to 2.5% (on dried substance) |
2.0% |
|
Sodium content |
11.3%-13.5%(on dried substance) |
12.4% |
|
SO42-/COO- |
≥1.8 |
2.4 |
|
Benzyl alcohol |
≤0.1%(m/m) |
0.02% |
|
Residual solvents |
DMF ≤880ppm |
Below detection limit |
|
Ethanol≤5000ppm |
891ppm |
|
|
Anti-factor Xa activity |
90IU to 125 IU/mg(on dried substance) |
981 IU/mg |
|
Anti-factor IIa activity |
20.0IU to 35.0IU(on dried substance) |
28.8IU/mg |
|
Micro organisms |
A: TAMC: ≤ 100CFU/g TYMC: ≤ 10CFU/g |
< 10CFU/g <10CFU/g |
|
B: Specified micro organisms Escherichia coli Salmonella |
Negative Negative Negative |
|
|
Free sulfate content |
≤0.50% |
0.11% |
|
Conclusion |
The sample complies with the requirements of EP10.5. |
|
|
Usage |
What is Enoxaparin sodium?
Enoxaparin sodium is a new anticoagulant. It is used for various indications including prevention of deep vein thrombosis (DVT). Enoxaparin sodium is a hemodilution drug prepared from heparin sodium, and its active ingredient is a mixture of natural polysaccharides.
Enoxaparin sodium is intended for use in the treatment of deep vein thrombosis; prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction, and treatment of acute deep vein thrombosis. Besides, Enoxaparin Sodium also can be used as a biomarker for diagnosis of atrial fibrillation cause of stroke.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


