PharmaSources/YiMarch 30, 2022
Tag: CAR-T therapy , CAR-T therapy Price , CAR-T therapy Approval
Currently, six CART-cell immunotherapies have been approved in China and abroad, as shown in the table below. Except for Abecma, the other five therapies are all targeted at CD19.
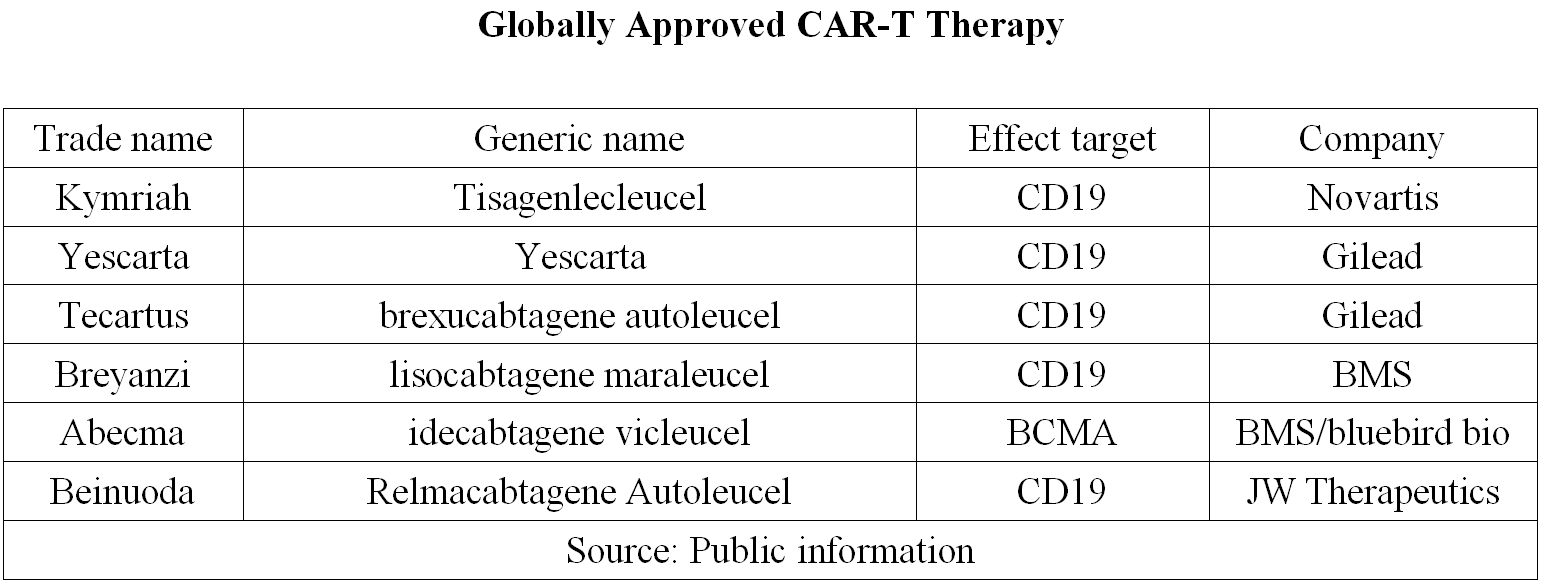
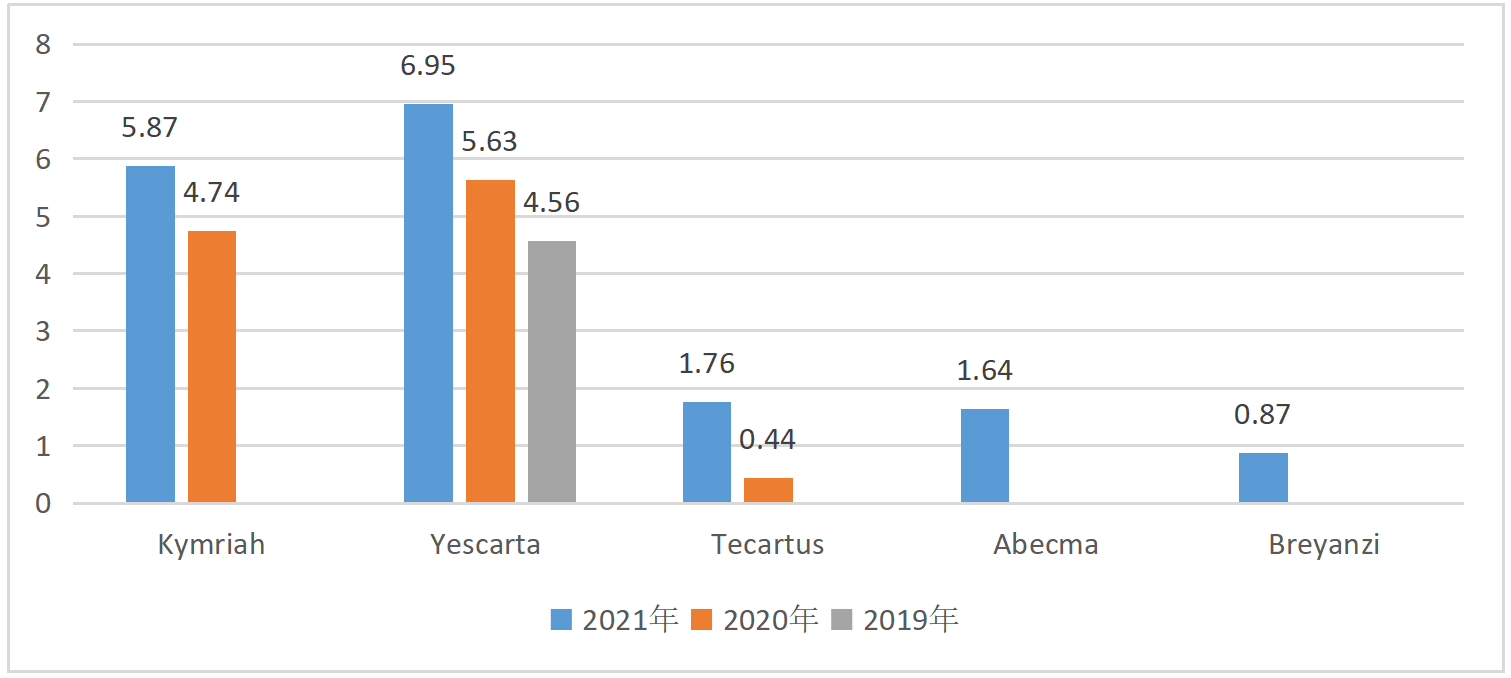
With the announcement of the company's financial report, the sales volume of the above five CAR-T therapies in 2021 also came out along with the companies' financial reports. Relmacabtagene Autoleucel is CART-cell immunotherapy developed by JW Therapeutics based on Juno's JCAR017 (i.e. Breyanzi), approved by NMPA in China in September 2021 to treat relapsed or refractory large B-cell lymphoma in adult patients after second-line or above systemic therapy.
Sales of Marketed CAR-T therapies (USD 100 million)
The CAR-T therapy market size is expanding from 2019 to 2021, with CAR-T therapy market sizes of USD 734 million, USD 1.081 billion, and USD 1.709 billion in these three years, respectively. And according to Frost & Sullivan's report: From 2019 to 2024, the compound annual growth rate of CAR-T therapy market size is 55.0%, and the market size will increase to USD 6.6 billion in 2024; The compound annual growth rate from 2024 to 2030 is expected to be 22.1%, and will further increase to USD 21.8 billion in 2030. It shows that the current CAR-T market is far from saturated and needs to be tapped by companies.
Back in 2021, many CAR-T therapies have made new progress in supervision:
Approved by the FDA in February 2021 for the treatment of adult patients with relapsed or refractory large B-cell lymphoma (R/R LBCL) who have received two or more systemic therapies;
Approved in Japan in March 2021 for the treatment of relapsed or refractory large B-cell lymphoma (R/R LBCL) and relapsed or refractory follicular lymphoma (R/R FL).
FDA approved Abecma in March 2021 to treat adult patients with relapsed/refractory multiple myeloma (R/R MM) that have received four or more therapies (including immunomodulators, protease inhibitors, and anti-CD38 antibodies).
Abecma received EC conditional approval in August 2021 to treat adult patients with R/R MM that have previously been treated at least three therapies (including immunomodulators, a protease inhibitor, and an anti-CD38 antibody) with disease progressed during treatment with the previous therapies.
Want to know more about CAR-T therapy and other medical and surgical supplies? Contact with Pharmasources!
Approved by the FDA in October 2021 for the treatment of adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL)
Approved in Japan in January 2021 for the treatment of certain adult patients with relapsed/refractory large B-cell lymphoma (LBCL)
The FDA approved Yescarta in March 2021 to treat adult patients with relapsed or refractory (R/R) follicular lymphoma (FL) who have previously received two or more systemic therapies.
sBLA was submitted to the FDA in October 2021 for second-line treatment of adult patients with relapsed or refractory large B-cell lymphoma (LBCL).
In October 2021, FDA and EMA acceptance of its marketing application for a new indication for the treatment of adult patients with relapsed or refractory follicular lymphoma (r/r FL) who have previously received at least two therapies;
In addition, Carvykti (cilta-cel), a BCMA-targeted CAR-T therapy jointly developed by Janssen and LEGN.US, submitted BLA to the FDA in 2021 to treat adult patients with relapsed/refractory multiple myeloma (MM). It is expected that with the approval of new indications and new products, the CAR-T therapy market will further expand in the future.
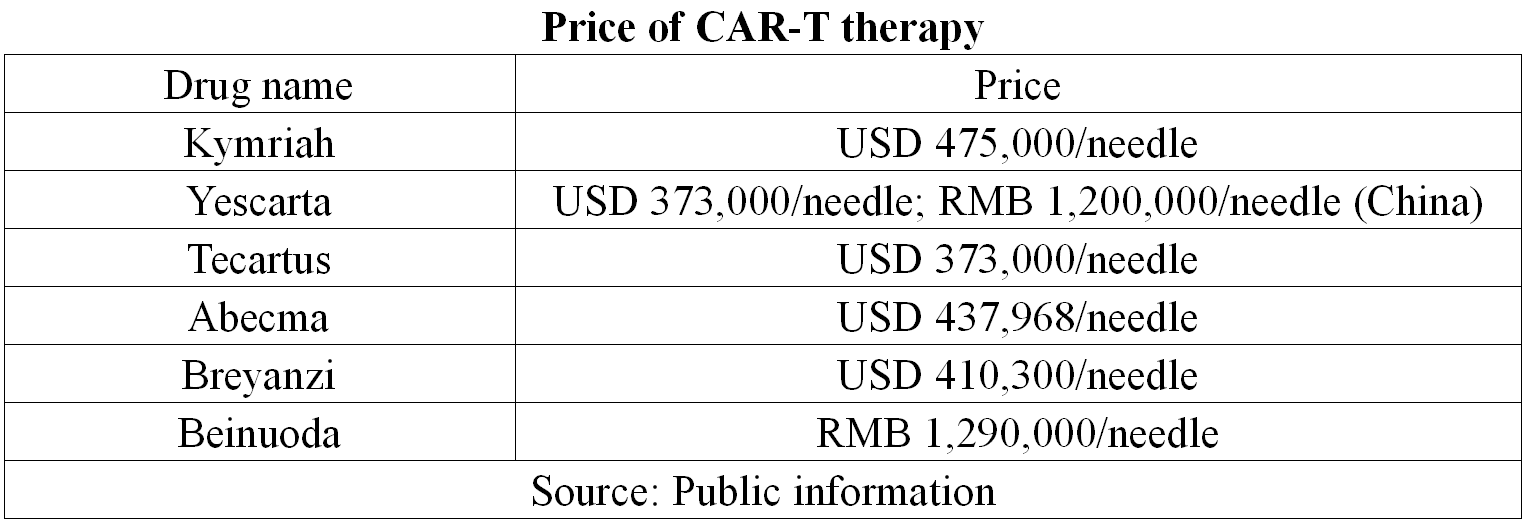
However, the price solution must be resolved for CAR-T therapy to reach more patients. At present, the price of CAR-T is sky-high, and the cheapest one is over 1 million yuan.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


