PharmaSources/YiMarch 30, 2022
Tag: Transaction , Vabysmo , Teclistamab , Bispecific Antibody
So far this year, there has been good news on the bispecific antibody therapeutics market.
· In mid-January, Johnson & Johnson submitted a BLA to the FDA for BCMA/CD3-targeted bispecific antibody teclistamab to treat patients with relapsed or refractory multiple myeloma (MM).
· On January 31, Roche's Vabysmo (faricimab-svoa), an Ang-2/VEGF-A-targeted bispecific antibody, was approved by the FDA to treat patients with neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME).
· On February 17, ZW25, a HER2-targeted bispecific antibody introduced by BeiGene from Zymeworks, was proposed by CDE to be included as a breakthrough therapy. The indication was monotherapy for HER2-positive locally advanced unresectable or metastatic biliary tract cancer (BTC) with failed systemic chemotherapy.
The bispecific antibody is a popular area of pharmaceutical R&D, with five varieties approved globally (see the table below for details, of which Removab exit market in 2017 due to poor sales after marketing.) The marketed bispecific antibody drugs have a broad range of indications, covering oncology, rare diseases, ophthalmic diseases, etc.
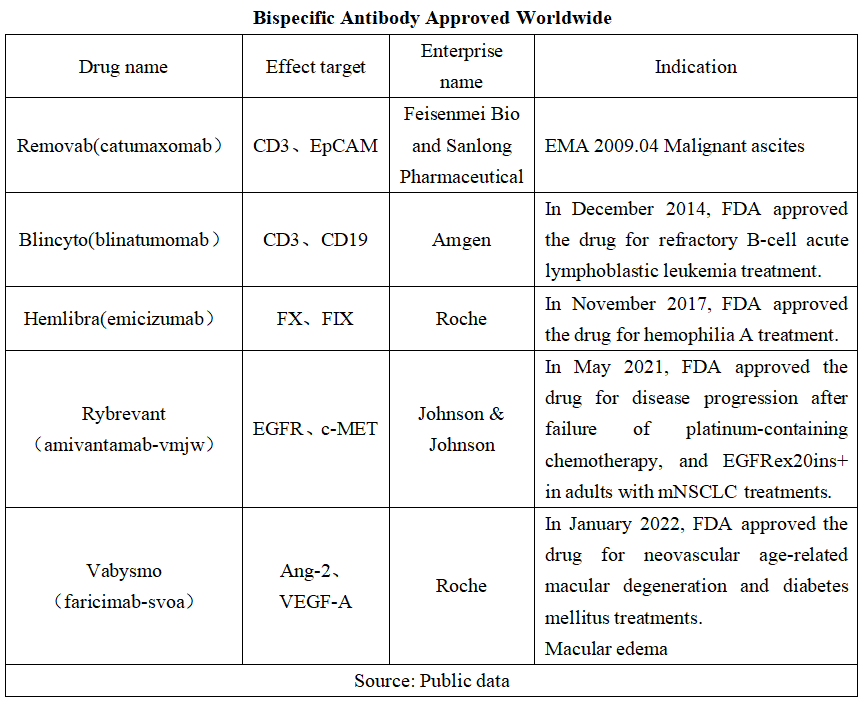
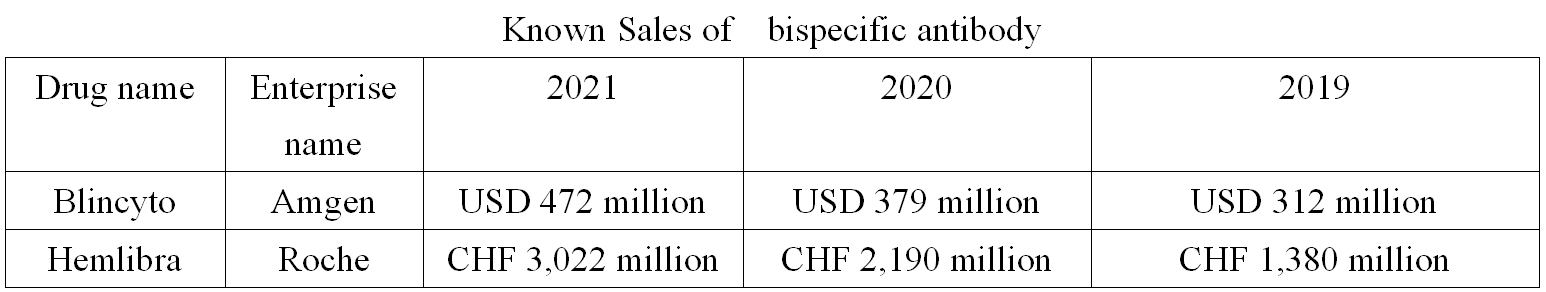
In terms of the sales volume we know, the global bispecific antibody market has been growing in recent years, with Roche's Hemlibra being a heavyweight product with a sales volume of over USD 3 billion.
Besides, there are many other bispecific antibodies in the late stage of clinical development, with at least 8 in Phase III and 25 in Phase II. According to public information, Chinese bispecific antibodies under research are mainly for tumors treatment. Nevertheless, these bispecific antibodies have very diverse targets, with PD-L1/4-1BB, EGFR/MET, CD3/CD20, and others being popular targets. Want to know more abotu bispecific antibody and medical supplies for sale? Contact with Pharmasources!
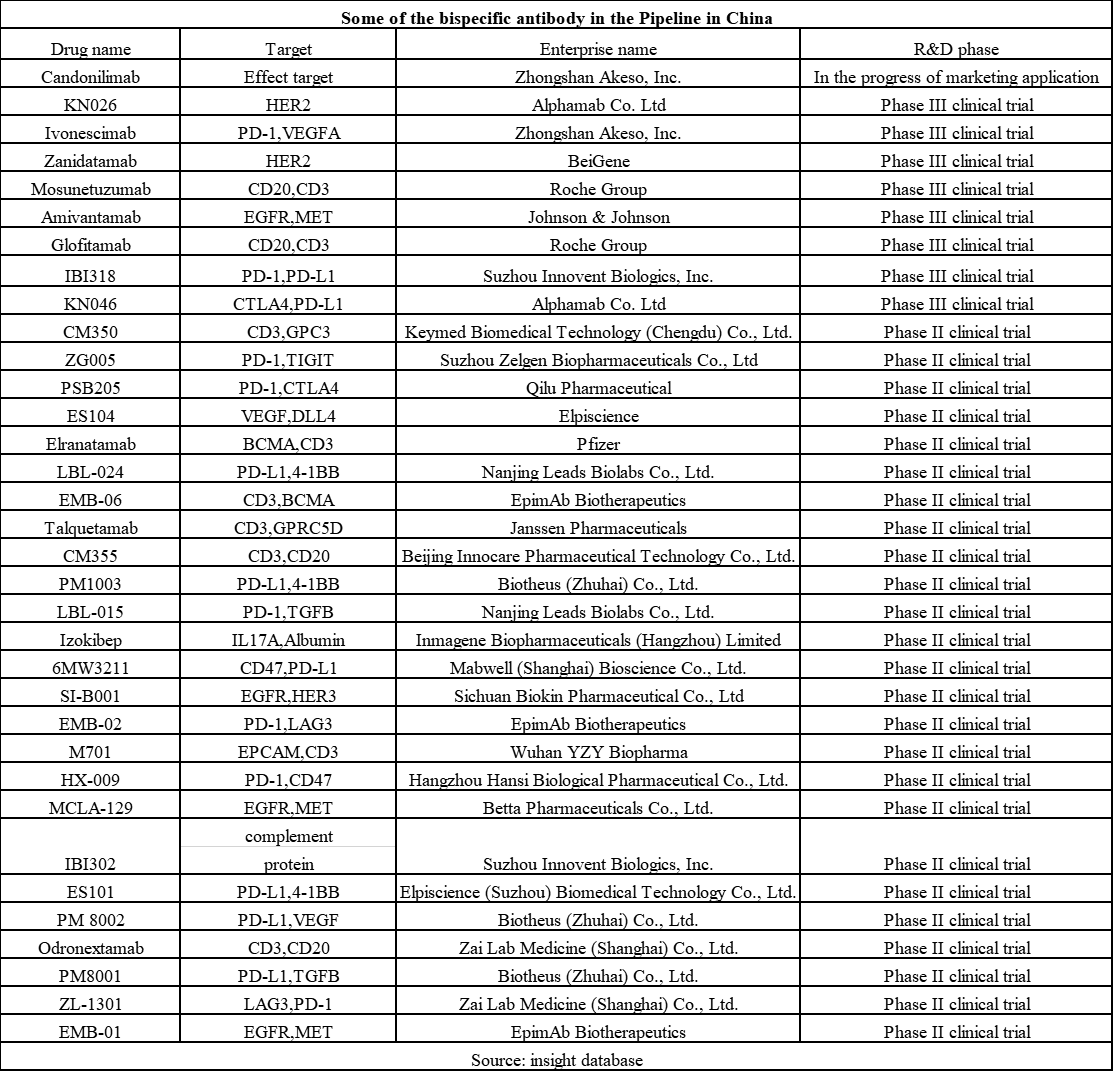
The same to the global bispecific antibody field, in which the competition is unquestionably more fierce. As the current progress, there will be explosive growth in the bispecific antibody market in the years to come. Some institutions predicted that its global market size will reach USD 80 billion in 2030, while the size of the Chinese market will hit USD 10.8 billion.
Furthermore, to capture the bispecific antibody market, Sanofi entered into an exclusive global collaboration and licensing agreement of over USD 1 billion with South Korea's ABL Bio in January 2022. They will jointly develop and commercialize ABL301, an α-synuclein, and IGF1R-targeted bispecific antibody. The drug employs Grabody-B platform technology to enhance the blood-brain barrier (BBB) penetration of antibodies to treat Parkinson's disease and other potential indications.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


