Xiaobin/PharmaSourcesJune 06, 2023
Tag: HER2 ADC , FDA , Drug marketing
On May 15, Byondis released a document on its official website, which showed that the FDA sent a complete response letter (CRL) regarding its biological license application of SYD985 to treat HER2-positive unresectable locally advanced or metastatic breast cancer (mBC).
The FDA stated in the CRL that more information is needed to support the approval decision, and additional time is also required for information review. While feeling regretful for FDA's decision, Byondis also presented its effort to keep advancing the marketing of SYD985 in the EU and England.
To overcome the shortcomings of poor plasma stability and narrow treatment window of the previous generation of HER2ADC (T-DM1), SYD985 has made improvements in both linker and toxin payload. SYD985 is a next-generation HER2 ADC, which is developed by Byondis using its proprietary linker-drug technology platform, ByonZine, based on duocarmazine. It is composed of trastuzumab and cleavable linker-drug valine-citrulline-seco-Duocarmycin-hydroxyBenzamide-Azaindole (vc-seco-DUBA).
After binding to HER2 on the surface of cancer cells, SYD985 is internalized by the cells. Subsequently, the linker breaks under the action of proteases and releases the cytotoxic drug (CD), seco-DUBA, which can bind to DNA minor grooves and destroy cell and nucleic acid structures, ultimately leading to tumor cell death. SYD985, designed through the ByonZine technology platform, is highly stable in blood circulation. If the CD is released early, it will quickly start self-destruction, reducing the damage to normal tissues and expanding the treatment window.
To reduce side effects, the drug-to-antibody ratio (DAR) of SYD985 is set lower at only 2.8. Although it affects the striking effect, SYD985 has a bystander effect, which to some extent improves the therapeutic effect.
However, the marketing of the innovative HER2 ADC was rejected by the FDA. To investigate the reasons behind it, we shall look through the clinical data.
The Biologicals License Application (BLA) of SYD985 was mainly based on its critical phase III TULIP research results. It was a randomized, multicenter, open-label clinical trial (n=436), which compares the efficacy of SYD985 and the regimen selected by doctors (PC Regimen) in the treatment of patients with unresectable HER2+ locally advanced or metastatic breast cancer. The primary endpoint of the research was progression-free survival (PFS).
The results showed that compared with the PC group, the PFS of patients in the SYD985 group was significantly prolonged (7.0 months vs 4.9 months; HR=0.64; P=0.002), reaching the primary endpoint. In terms of the overall survival (OS), which was the secondary endpoint, the SYD985 group was 20.4 months, and the PC group was 16.3 months. Although SYD985 presented an improvement in survival for nearly four months, as the P value was 0.153, the OS improvement for patients from SYD985 was not significant compared to the PC group.
Regarding safety, 52.8% and 48.2% of patients in the SYD985 group and the PC group experienced level 3 and above adverse events, respectively. Three patients (1%) in the SYD985 group experienced any level of interstitial lung disease (ILD), with one patient experiencing level 3 and above adverse event.
Let's check the clinical data of DS-8201, which was regarded as the "ceiling" in the HER2 ADC field. In the DESTINY-Breast02 (DB02) trial targeted at patients with HER2-positive unresectable and metastatic breast cancer, the PFS (primary endpoint) of the DS-8201 group and the PC group was 17.8 months and 6.9 months, respectively; and the OS (secondary endpoint) of the DS-8201 group and the PC group was 39.2 months and 26.5 months, respectively.
After comparison, it is obvious that DS-8201 has almost changed the second-line and even the overall treatment pattern of HER2+ breast cancer. Therefore, if SYD985 wants to get a piece of the action, it is necessary to provide data with significant differences, which is apparently an unrealizable goal at present.
HER2 belongs to the epidermal growth factor receptor (EGFR) family and is expressed in about 15% - 20% of breast cancer patients. Meanwhile, HER2 is a protooncogene that can cause the division and proliferation of cancer cells. HER2 is the preferred target of ADC because its expression level differs greatly between various tumor cells and normal histiocytes and better receptor-mediated endocytosis.
At present, three HER2 ADCs have been approved for marketing globally, they are Kadcyla (T-DM1, ado-trastuzumab emtansine) developed by Roche, Enhertu (DS-8201, T-Dxd, trastuzumab deruxtecan) developed by Daiichi Sankyo/AstraZeneca, and RC48 ( disitamab vedotin) developed by Remegen, and the approved indications involve breast cancer, gastric cancer, urothelial carcinoma, etc.
Compared with foreign countries, the R&D enthusiasm of HER2 ADC in China runs high. At present, multiple HER2 ADCs have entered the clinical stage in China.
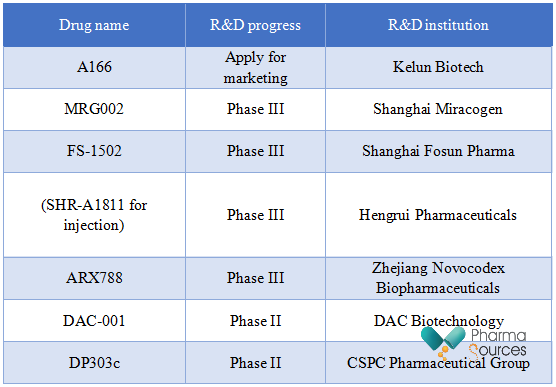
Among them, the marketing application of A166 developed by Kelun Biotech has been accepted by NMPA recently, and the proposed indication is HER2+ unresectable locally advanced, recurrent, or metastatic breast cancer that has previously failed in the second-line and above anti-HER2 treatment.
At the 2022 ASCO annual meeting, Kelun Biotech announced the data of the phase I dose expansion study of A166 for HER2+ breast cancer patients undergoing multi-line treatment: The ORR of the 4.8 mg/kg group reached 73.9%, and the median PFS reached 12.3 months. Currently, A166 is undergoing multiple clinical trials.
ARX788 is a next-generation anti-HER2 ADC that uses unnatural amino acid technology to achieve targeted coupling. The recently published phase III clinical research of HER2+ breast cancer (ACE-Breast-02) reached the predetermined medium-term primary efficacy endpoint. Compared with capecitabine and lapatinib, PFS showed a statistical difference. The effective rate of ARX788 is about 25% for patients who use DS-8201 within 12 months and present drug resistance. Based on this, Novocodex Biopharmaceuticals proposes to communicate with the CDE to apply for early termination of the study and submit a new drug application.
SHR-A1811 is composed of trastuzumab, a cleavable tetrapeptide linker, and SHR9265, a novel topoisomerase I inhibitor payload. It presents HER2-dependent growth inhibition in a variety of breast cancer and gastric cancer cell lines and has a bystander effect. At the 2023 AACR meeting, Hengrui Pharmaceuticals announced the results of the global multicenter phase I clinical trial of SHR-A1811. Among 250 patients who had previously received frontline treatment with a median of 3 in a metastatic disease state, the ORR was 61.6% (95%CI 55.3-67.7), and the 6-month PFS rate was 73.9%.
At present, SHR-A1811 is in phase III clinical trial, targeting HER2+ breast cancer and HER2 low expression breast cancer respectively.
The emergence of DS-8201 has made HER2 ADC latecomers need extraordinary strength to be approved for marketing. The FDA's rejection of the marketing of SYD985 is just the beginning, and with the rising enthusiasm for the R&D of ADCs, the standards of subsequent review by the FDA of the popular target ADCs will be inevitably raised. In the future, only strong ADCs with better safety, more diverse indications, or excellent therapeutic effects in combination therapy can break out of the competition. It is hoped that Chinese pharmaceutical companies can make real innovative modifications in antibodies, linkers, and toxins, explore more untapped areas, and create greater clinical value.
http://www.cninfo.com.cn/new/disclosure/detail?stockCode=002422&announcementId=1216795846&orgId=9900012788&announcementTime=2023-05-11;
Tarantino P, Hamilton E, Tolaney SM, et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J Clin Oncol. 2020;38(17):1951-1962. doi:10.1200/JCO.19.02488;
History is a mirror that reflects the rise and fall. Where will ADCs Come and Go In the Post DS-8201 Era?, East Asia Qianhai Securities, June 22, 2022;
Quanxia Lv, Ziyuan Meng, Yuanyuan Yu, Feng Jiang, Daogang Guan, Chao Liang, Junwei Zhou, Aiping Lu and Ge Zhang, Molecular Mechanisms and Translational Therapies for Human Epidermal Receptor 2 Positive Breast Cancer, Int. J. Mol. Sci. 2016, 17, 2095.
Xiaobin holds a Master's degree in Pharmacy and currently work as a public health control staff. Navigating through the intricate and complex data each day and feeling a sense of insignificance of herself. While being happy to witness the golden time of the development of Chinese bio-pharmaceutical industry. Hope to learn and improve together with everyone.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


