David Orchard-WebbMarch 01, 2022
Tag: Cell Therapy , Oncology , Inhibitor
So far in the first half of Q1 2022 seven biopharma companies have IPO’d on the NASDAQ. They are focused on oncology, cardio-renal diseases, neurodegenerative diseases, and cell therapy. Figure 1 shows the global pharmaceutical market share of these indications as of 2020.
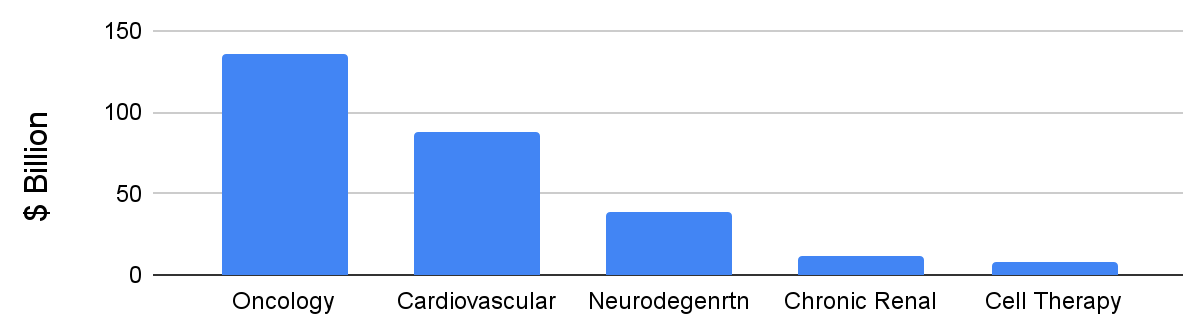
Figure 1: 2020 global market share vs. indication.
There is some overlap between cell therapy and oncology, for example two of the four oncology focused companies that IPO’d in 2022 are also cell therapy companies. Two companies specialize in neurodegenerative diseases, and one company treats both cardiovascular and renal disease, which may result secondarily.
Hillstream is at the cutting edge of oncology drug development, taking a new class of therapeutic candidates into the clinic targeting ferroptosis, an emerging mechanism resulting in iron mediated cell death (IMCD) of drug resistant cancers.
Hillstream’s Chief Executive Officer (CEO) is Randy Milby, who previously held a Board of Directors position at CorMedix, developing and commercializing drugs for inflammatory and infectious diseases. Inflammation is one of the hallmarks of cancer, putting Mr. Milby in a great position to direct Hillstream’s development.
The very experienced Robert Niecestro, PhD, heads Regulatory Affairs. Notably, he previously served as Senior Director, Clinical Development and Therapeutic Head for Gastrointestinal, Oncology and Stroke at Eisai Inc., amongst others. Dr. Niecestro has contributed to filing of over 60 INDs, and has gained drug marketing approval through 13 NDAs/ BLAs in the United States. His NDA/BLA to IND ratio (22%) is exceptional, considering that according to the trade association Biotechnology Innovation Organization (BIO) the overall likelihood of drug approval from IND to market between 2011 to 2020 was only 8%. The leadership also includes experienced translational/ manufacturing/ clinical/ regulatory personnel as well as a data science position.
Their most advanced product candidate is HSB-1216, a nanoparticle formulation of salinomycin derived from the company’s PLA/ PEG Quatramer technology, which has several benefits compared to free salinomycin. Salinomycin is a potent cancer stem cell inhibitor and IMCD inducer. It is also an antibiotic. In a clinical pilot study conducted in Germany by the University of Heidelberg, salinomycin was found to reduce tumor burden in treatment resistant cancers, including triple negative breast cancer and epithelial carcinomas.
The company has developed Trident Artificial Intelligence (TridentAI), a precision medicine platform, that is used to identify biomarkers in their clinical programs that represent specific patient subgroups. This ability may greatly improve their chances of navigating clinical trials to market approval.
Nuvectis are focused on developing targeted therapies for cancer. Their two lead candidates are tablet formulated inhibitors of the Heat Shock Factor 1 (HSF1) pathway and the SRC/YES1 kinase.
Ron Bentsur serves as Chairman, CEO and President, Enrique Poradosu, PhD is Executive Vice President, Chief Scientific and Business Officer, and Shay Shemesh is Executive Vice President & Chief Development Officer. The team has extensive experience in licensing promising product candidates.
The company is focused on a “tumor-agnostic” approach, that is they believe the mutational signature of a given cancer is more important than its anatomical location for selecting a treatment. They are developing their two lead candidates with this in mind and intend to use a biomarker-based patient selection approach for clinical trials and beyond.
NXP800 (HSF1 inhibitor) entered the clinic in December 2021. Once phase Ia is completed the phase Ib will further assess safety and begin evaluating the preliminary anti-tumor activity of NXP800 in biomarker-selected patients, initially in ovarian clear cell carcinoma and endometrioid carcinoma.
Arcellx is a clinical-stage biotechnology company engineering innovative immunotherapies for patients with cancer and other incurable diseases. They have developed the D-Domain (dd) , a small, stable, fully synthetic binding agent with a hydrophobic core for use with CAR-T and other T-cell targeting scaffolds like the company’s SparX.
Rami Elghandour is the Chairman and CEO, and David Tice, PhD is the Chief Scientific Officer.
Eleven other leadership positions are held by experienced personnel.
High unmet clinical need is the focus of Arcellx’s cell therapy program. This includes treatments for multiple myeloma, acute myeloid leukemia, myelodysplastic syndrome, and solid tumors. The pipeline is ambitious, starting with a phase I clinical trial delivering CART-ddBCMA to patients with relapsed or refractory multiple myeloma (r/r MM).
Consistent with the observations in an ongoing Phase I trial of CART-ddBCMA, the technology allows a high proportion of CAR+ cells, lowering the total number of T cells required to be administered which yields a therapy with an improved toxicity profile. For 19 evaluable patients the overall response rate (ORR) was 100% and the duration of response (DOR) was more than half of patients with r/r MM having ongoing responses for at least one year following treatment.
TC BioPharm is a clinical-stage cell therapy company developing advanced allogeneic CAR-T cell therapy products for the treatment of cancer, as well as developing gamma delta T cell therapies for the treatment of infectious disease. Established in 2014, the company has expanded into 5 global locations and grown to over 100 employees.
Bryan Kobel is the CEO, Dr. Michael Leek is Co-Founder And Executive Chairman, Angela Scott is Co-Founder & Chief Operating Officer, Dr Sebastian Wanless is Senior Clinical Director. Three other leadership positions are held at the company.
OmnImmune® is the company’s unmodified allogeneic gamma delta T cell product, being initially in phase I clinical trial for the treatment of Acute Myeloid Leukemia (AML) patients who have not responded well to first-line therapy with the aim of delaying or preventing the need for human bone-marrow transplant.
The company also has a number of in-house and partner programs at the preclinical stage focussed on developing CAR modified allogeneic gamma delta T-cell products targeting solid and hematological indications.
CinCor is a clinical-stage bio pharmaceutical company highly focused on developing its clinical candidate CIN-107 for the treatment of hypertension and other cardio-renal diseases.
Marc De Garidel with previous experience at Eli Lilly & Co and Amgen is the CEO, Catherine Pearce, DHSC, is the COO & Co-Founder. The company has two other executive positions.
An oral small molecule inhibitor, CIN-107 is highly selective for aldosterone synthase, the enzyme responsible for the synthesis of aldosterone in the adrenal gland. The goal of providing an improved treatment for patients suffering from hypertension, or high blood pressure.
CinCor is conducting phase II clinical trials in hypertension and primary aldosteronism. The company plans to initiate a Phase II clinical trial in 2022 to explore its utility in ameliorating complications of chronic kidney disease. Earlier this year, we initiated a Phase 2 clinical trial of CIN-107 in patients with confirmed PA, which we refer to as our spark-PA trial, and plan to initiate a Phase 2 clinical trial in patients with CKD who have uncontrolled blood pressure in the first half of 2022.
Vigil harnesses microglia, the sentinel cells of the brain’s immune system, to improve the lives of patients, caregivers, and families affected by rare and common neurodegenerative diseases by restoring their vigilance.
Ivana Magovčević-Liebisch, PhD, JD, an accomplished pharma and biotech executive, is the President & CEO, Evan Thackaberry, PhD, DABT is Sr Vice-President, Early Development, having held previous positions at Ra Pharmaceuticals, Genentech, and Schering-Plough. There are two other management positions at Vigil.
VGL101, is a fully human monoclonal antibody targeting human TREM2 for the treatment of rare microgliopathies. Safety is currently being evaluated in a phase I trial with a healthy cohort. The initially selected indication is adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP), a rare, genetically defined, and fatal neurodegenerative disease caused by microglial dysfunction. TREM2 may compensate for other losses of function within the microglia.
Vigil is also developing small molecule agonists of TREM2 for use in more common neurodegenerative conditions such as Alzheimer’s. Small molecules have the advantage of oral availability.
Amylyx’s mission is to develop therapies that change the treatment paradigm for amyotrophic lateral sclerosis (ALS) and a broad range of neurodegenerative diseases by keeping neurons alive.
Josh Cohen and Justin Klee are Co-CEO and Co-Founder, Patrick Yeramian, MD, MBA is Global Head of Clinical R&D & CMO.
AMX0035 is a dual unfolded protein response (UPR)-Bax apoptosis inhibitor composed of sod-ium pheny-lbuty-rate and tauroursodeoxycholic acid (TUDCA). Through the resolution of UPR and by inhibiting translocation of Bax, to the outer mitochondrial membrane, it has been shown in multiple models that AMX0035 can keep neurons alive under a variety of different conditions and stresses.
A US marketing application, the New Drug Application (NDA) was filed in Q4 2021 for the treatment of ALS with AMX0035. The equivalent has also been filed in Canada. Both these applications were made on the basis of successful phase II trials, no phase III have been performed. The applications are currently under review.
In the first half of Q1 the pipeline of biopharmaceutical IPOs listed on the NASDAQ is going strong in 2022, with seven, innovative, newly publicly listed companies aiming to treat the disease indications of oncology, cardiovascular disease with renal complications, and neurodegeneration using small molecules as well as biologics including antibodies and cell therapies.
David Orchard-Webb, Ph.D., is a technical writer with broad interests including health & technology writing, plus extensive training and knowledge of biomedicine and microbiology. My Ph.D. and postdoc were in oncology and developing cancer medicines. I provide technical medical and other writing services for projects ranging from “knowledge automation” to pure pharma, to food safety, to the history of science, and everything in between. I also provide white papers, ebooks, meta-analysis reviews, editing, consulting, business, and market research-related activities in biomedicine, technology, and health. In addition to its well-known role in the development of medicines, I am a big believer in biotechnology’s ability to revolutionize industries such as food-tech, agtech, textiles & fashion.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


