zhulikou431/PharmaSourcesJuly 12, 2023
PIC/S in Chinese is Pharmaceutical Inspection Co-operation Scheme. In October 1970, 10 nations in EFTA, including Austria, Denmark, Finland, Iceland, Liechtenstein, Norway, Portugal, Sweden, Switzerland, and United Kingdom, established Pharmaceutical Inspection Convention (PIC) and signed the first Mutual Recognition Agreements (MRAs) of drug GMP inspection so as to eliminate drug trade barriers and promote the coordination and unification of drug GMP; It is suitable for the origin and foundation of PIC/S organization today.
In September 2021, China NMPA started the work to join PIC/S. According to the work processes of PIC/S, there are 2 phases to join PIC/S: Phase I is the pre-evaluation phase, taking for 2 years; Phase II is the formal evaluation phase, taking for about 6 years. At present, China NMPA is in the pre-evaluation phase. PIC/S updated the GMP guideline recently, entering a new stage. This article will introduce China NMPA's progress in joining PIC/S and the latest change in GMP guideline of PIC/S respectively.
China NMPA's main progress in joining PIC/S
The following table summarizes the progress involving PIC/S released by the pharmaceutical authority in China since September 2021.
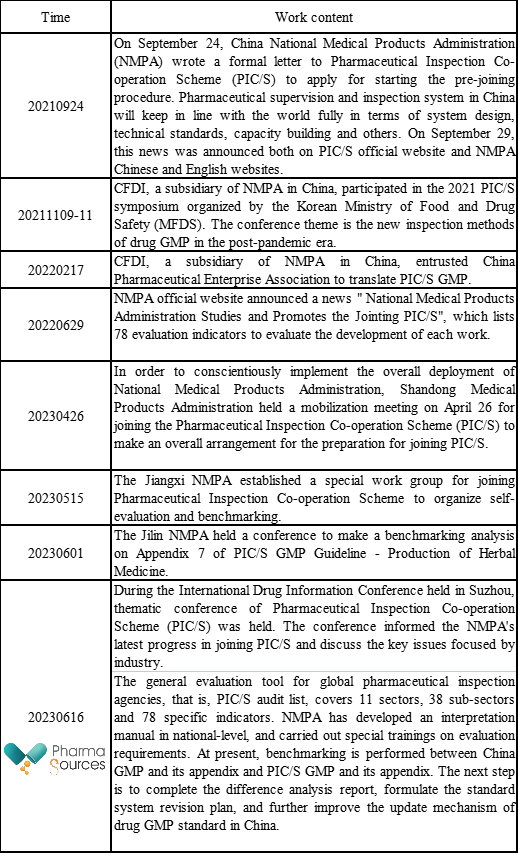
1- "Comparative Analysis of China GMP and PIC/S GMP"
2- Information on PIC/S official website
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


