PharmaSources/Small pillMarch 16, 2022
Tag: CAR-T , FDA , multiple myeloma
On February 28 local time, Legend Biotech officially announced in Somerset, New Jersey, U.S., that its self-developed BCMA-targeted CAR-T therapy, ciltacabtagene autoleucel (trade name: CARVYKTI®), abbreviated as Cilta-cel, was approved for marketing by the FDA. It is for the treatment of adult patients with Relapsed/Refractory Multiple Myeloma (R/R MM) who have received four or more prior therapies, including proteasome inhibitors, immunomodulators and anti-CD38 monoclonal antibody. This is the first original Chinese CAR-T drug approved for marketing in the U.S., representing a new level of internationalization for Chinese innovative drugs.
Beyond the seas, the Biologics License Application (BLA) was submitted by Janssen, a partner of LEGN.US. In May 2021, the FDA accepted the BLA of Cilta-cel and granted priority review status with a PDUFA date of November 29. In early November, LEGN.US announced that the FDA delayed the review to February 28, and today, it was finally approved.
Multiple Myeloma (MM) is a malignant tumor caused by the malignant proliferation of plasma cells in the bone marrow and is considered an incurable neoplastic hematologic disorder. According to the American Cancer Society, it is estimated that more than 34,000 people in the U.S. will be diagnosed with Multiple Myeloma in 2022 and more than 12,000 will die from it. While some patients with Multiple Myeloma have no obvious symptoms, in most cases, patients are diagnosed due to the presence of associated symptoms, which may include bone diseases, abnormalities of low blood counts, elevated blood calcium, kidney diseases or infections. Although some symptoms may be relieved with treatment, unfortunately, it is likely to relapse. Patients whose disease relapses after treatment with standard therapies, including protease inhibitors, immunomodulators, and anti-CD38 monoclonal antibody, will face a poor prognosis and limited treatment options.
BCMA (B-cell Maturation Antigen) is a transmembrane glycoprotein. It is part of the Tumor Necrosis Factor (TNF) receptor superfamily, which is also known as TNFRSF17 or CD269. Researches have revealed that BCMA plays a key role in the proliferation and survival of B cells and is expressed primarily in plasma cells and mature B lymphocytes. Scientific evidence has now shown that BCMA is over expressed in Multiple Myeloma and shows an increase in expression with disease progression, thus, BCMA can be an ideal target for treating it.
Cilta-cel is a chimeric antigen receptor T-cell therapy (CAR-T) targeting BCMA. The therapy contains a 4-1BB co-stimulatory structural domain and two BCMA-targeted single-domain antibodies, which is designed to increase its comprehensive ability to target cancer cells.
The FDA approval was based on the results of a pivotal Ib/Phase II CARTITUDE-1 research that evaluated the efficacy and safety of Cilta-cel in patients with Relapsed/Refractory Multiple Myeloma.
Among 97 subjects recruited, 99% were ineffective on last-line therapy, and 88% presented unresponsiveness after receiving at least 3 lines of prior therapy, including immunomodulators, proteasome inhibitors, and anti-CD38 antibodies. According to the research, at a median follow-up of 12.4 months, the Objective Response Rate (ORR) reviewed by the independent commissioners was 97%, including 67% of sCR (stringent complete response), and 92.8% of very good partial response (VGPR) and above. The incidence rate of ≥ Grade 3 Cytokine Release Syndrome (CRS) was 4% and that of ≥ Grade 3 neurotoxicity was 9%.
At the latest ASCO annual meeting in 2021, the ORR for patients treated with Cilta-cel has reached 97.9%, according to the updated 18-month median follow-up data from the CARTITUDE-1 research by LEGN.US.
According to the preliminary data from the CARTITUDE-2 research cohort A, the ORR of MM patients who have received previous first-to-third-line therapy is 95%, of which 75% achieves sCR/CR.
Cilta-cel has a unique drug structure and significantly lower clinical dose than other comparable CAR-T products, with high safety and excellent efficacy when using.
Want to get more information on Cilta-cel and other kinds of medical hospital supplies? Pharmasources could help you!
Cilta-cel received its first approval for Investigational New Drug (IND) CAR-T from the National Medical Products Administration in 2018, and received IND approval from the FDA in the same year, followed by the FDA's identification as a breakthrough therapy and an orphan drug, identification as one of the European Medicines Agency Priority Medicines (PRIME) and identification by FDA as a breakthrough therapy in 2019, and the first identification as a "breakthrough therapy" in China in August 2020. In December 2020, LEGN.US made a rolling submission of BLA to the FDA for Cilta-cel. In the same month, Janssen also submitted a New Drug Application (NDA) for Cilta-cel to the Japanese Ministry of Health, Labour and Welfare.
Currently, the marketed and investigational CAR-T therapies are heavily stacked on the CD19 target, followed by BCMA, but with relatively less competition. There are currently seven CAR-T therapeutic products on the global market, namely Kymriah of Novartis, Yescarta and Tecartus of Gilead's subsidiary Kite, Breyanzi of Bristol-Myers Squibb, Abecma of Celgene, as well as Axicabtagene Ciloleucel Injection of Fosun Kite and Relmacabtagene Autoleucel Injection of JW Therapeutics, which were approved for marketing in China.
In March 2021, Abecma of Bristol-Myers Squibb (BMS) and Bluebird Bio was approved by the FDA, pre-empting the world's first CAR-T cell therapy targeting BCMA. Abecma is priced at US$ 419,500, and its first-year sales is US$ 164 million, about twice as high as the CAR-T products approved during the same period. Additionally, according to the report of Frost & Sullivan, the global CAR-T therapy market is expected to reach US$ 6.6 billion in 2024 and US$ 21.8 billion in 2030, and the global market is not yet saturated. And Cilta-cel, as the second approved CAR-T therapy targeting BCMA entering the global market, can refer to the sales volume of Abecma, its most direct competitor.
Now, LEGN.US' Cilta-cel is about to meet the harvest, and it will bring more treatment options for Multiple Myeloma patients and drive LEGN.US to take the first step of commercialization. The pricing of Cilta-cel is reported to be US$ 465,000, and not surprisingly, its marketing approval will bring a real boost to LEGN.US' performance and promising profit margins.
The therapy is currently available in both Chinese and foreign companies. In China, the pharmaceutical companies conducting the R&D of BCMA CAR-T therapy are progressing smoothly, except for the approved LEGN.US, CARsgen, Innovent/IASO Bio, Gracell Bio and Hrain Biotechnology are in the forefront of clinical research.
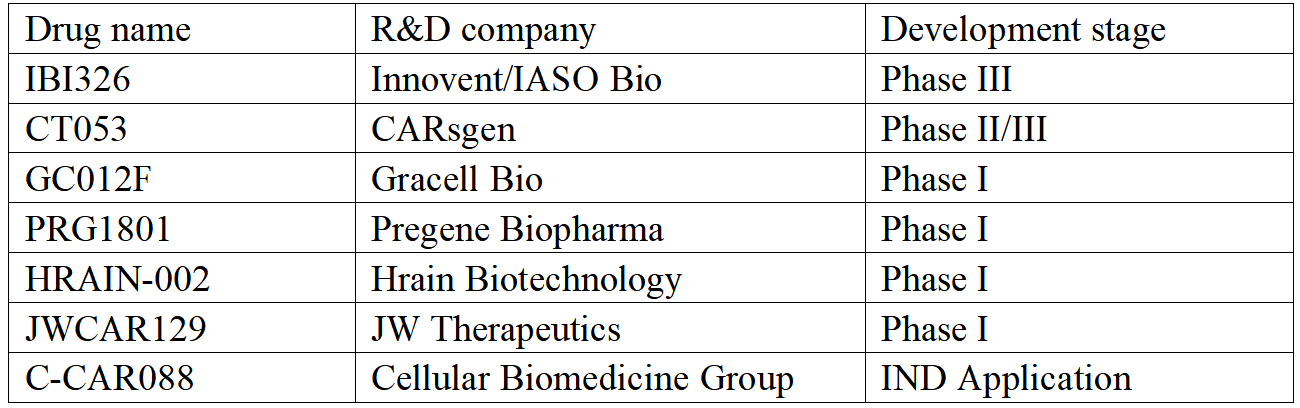
Some Chinese BCMA CAR-T therapy R&D companies (according to public information)
LEGN.US has globally submitted marketing applications to the FDA of the U.S., EMA of EU and PMDA of Japan in collaboration with Janssen, and is about to apply for marketing in China. According to the previous disclosures, the company plans to submit a marketing application to the CDE in early 2022. Moreover, Cilta-cel has been included in the CDE's list of breakthrough therapies, which means that the drug will be approved in China at an accelerated pace. Besides Cilta-cel, there are 8 other CAR-T cell therapies in LEGN.US' pipeline that have entered the clinical stage. Let's look forward to the next breakthrough of LEGN.US.
1. https://www1.hkexnews.hk/listedco/listconews/sehk/2022/0301/2022030100146_c.pdf;
2. Official website of LEGN.US.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


