Yi/PharmaSourcesJuly 10, 2023
Tag: AMP945 , FAK inhibitor
Recently, Amplia Therapeutics Limited announced its FAK inhibitor AMP945 under research combined with FOLFIRINOX was effective on the preclinical model of pancreatic cancer; Mice treated with AMP945 and FOLFIRINOX have a higher survival rate than those treated with FOLFIRINOX only.
AMP945, an oral focal adhesion kinase (FAK) inhibitor with a high selectivity, has been awarded the Orphan Drug Designation for pancreatic cancer and idiopathic pulmonary fibrosis by FDA. At present, AMP945 is in a Phase 1b/2a clinical trial, aiming to confirm whether it can improve the first-line therapeutic effect on patients with unresectable or metastatic pancreatic cancer to administer AMP94 before the gemcitabine and albumin paclitaxel.
FAK, that is, focal adhesion kinase, is a kind of non-receptor protein tyrosine kinase in the cytoplasm and a kind of adaptor protein mainly regulating adhesion signaling and cell migration, which can promote cell cycle, adhesion, metastasis and survival through kinase dependence and independence.
FAK is composed of N-terminal 4.1-Ezrin-Radixin-Moesin (FERM) structural domain, central kinase domain, and C-terminal FAT structural domain with 2 PRs (PR1 and PR2) and focal adhesion localization area. Among them, the central kinase domain plays an enzyme catalytic role. N-terminal FERM structural domain mediates the direct interaction between FAK and proteins such as integrin and growth factor receptor, and binds with central kinase domain directly to prevent the substrate from binding to the catalytic domain and protect FAK from phosphorylation of sarcoma protein kinase (Src). C-terminal FAT structural domain contains a variety of protein-protein interaction binding sites, guiding FAK to focal adhesion complexes in various cells.
FAK is activated by tyrosine kinase (RTK), intracellular pH change (H+), integrin, G protein-coupled receptor (GPCR) and cytokine receptor, and increases cell motility by affecting the signaling mediated by ARP2/3, RHOGEF, talin or cortactin, and SRC or PI3K.
Studies have found that FAK can overexpressed in many tumors, such as ovarian cancer, breast cancer, lung cancer, colorectal cancer, pancreatic cancer, prostate cancer and head and neck cancer, and it is related to the poor prognosis of many cancers. It can inhibit the proliferation, migration and invasion of tumor cells to inhibit and degrade FAK. However, researchers have found that to inhibit FAK signaling pathway can effectively reverse many failed chemotherapies and targeted therapies caused by tumor drug resistance, and can enhance the response and efficacy of immunotherapy for solid tumors.
But the R&D of FAK inhibitor is not successful. Although FAK inhibitor shows certain anti-tumor activity in some tumor types, single-drug has no obvious improvement in disease treatment in the research of various companies. Thus, enterprises that participated in the development of FAK inhibitors in the early stage chose to give up halfway, among which Pfizer transferred defactinib to Verastem, and Boehringer Ingelheim (BI) transferred IN10018 to InxMed.
With the in-depth study of FAK target and its action mechanism, there are many FAK inhibitors under research in global at present, as shown in the table below. The development of FAK inhibitor trends to drug combinations for treating many tumors. In addition to chemical drugs, FAK inhibitors under research also include FAK degradation agent, such as BI-3663 and BI-0319.
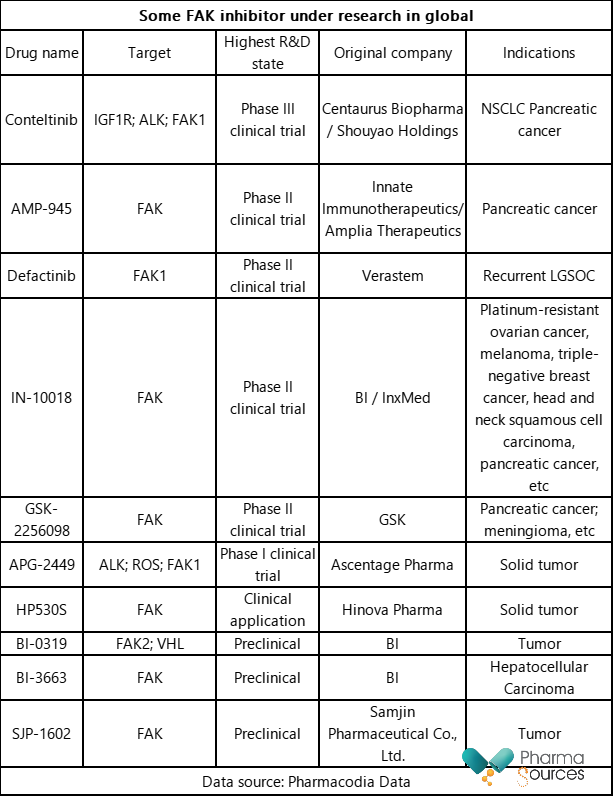
FAK inhibitors under research include some multi-target kinase inhibitors, such as Conteltinib and APG-2449. Wherein Conteltinib (CT-707) has a fast progress, and the clinical trial of its combination with crizotinib in the treatment of ALK-positive advanced NSCLC has entered Phase III.
Conteltinib is a multi-target small molecule kinase inhibitor, which can act on FAK, Pyk2 and ALK targets. In accordance with preclinical research: In many kinds of tumor cells, such as pancreatic cancer, CT-707 can effectively inhibit the kinase activity of FAK protein and block the FAK-related signaling pathway in tumor cells. In the animal tumor model, CT-707 combined with PD-1 antibody and gemcitabine can effectively inhibit the tumor of pancreatic cancer and improve the survival status of tumor-bearing mice. At present, Phase 1b/2 study is in progress, which evaluates the safety, tolerability, pharmacokinetics and efficacy of CT-707 combined with Toripalimab injection and gemcitabine in patients with advanced pancreatic cancer.
APG-2449 is a new small molecule FAK/ALK/ROS1 triple tyrosine kinase inhibitor with oral activity self-developed by Ascentage Pharma. In accordance with preclinical tumor model research: APG-2449 can overcome the drug resistance of the first generation ALK inhibitor and show a synergistic effect with EGFR inhibitors (especially Osimertinib) in the tumor model of NSCLC xenotransplantation with EGFR T790M mutation.
In the first human Phase I trial, APG-2449 treats the patients with second-generation TKI-resistant ALK/ROS1 + NSCLC and malignant mesothelioma. At the 2022 ASCO Annual Conference, it announced the trial data: In the second generation TKI drug-resistant patients and newly-treated patients, APG-2449 had a preliminary curative effect. Among the 14 cases of the second generation TKI drug-resistant patients with ALK positive NSCLC, 4 cases were partial response (PR); In the 10 cases of newly-treated ALK/ROS1 positive patients, ORR reached 80% and DCR was 100%.
Defactinib is an oral potent ATP competitive FAK inhibitor with a good tolerability. In the Part a of RAMP 201, the ongoing Phase II registration trial, it evaluates the safety and efficacy of RAF/MEK inhibitor avutometinib alone and combined with defactinib in the treatment of recurrent LGSOC. In May 2023, Verastor announced it had achieved positive results: The combination therapy of avutometinib and defactinib made an ORR of 45% (13/29) and a tumor shrinking rate of 86% (25/29) in the evaluated patients. Safety and tolerability keep good, consistent with previously reported data. It is worth mentioning that the combination therapy of avutometinib and defactinib has been granted a breakthrough therapy designation by the FDA for treating all the recurrent LGSOC patients (Regardless of KRAS status).
IN10018, an efficient and highly selective ATP competitive FAK inhibitor, has a good safety and effectiveness in treating various tumors, as shown in the early clinical data. The latest study found that in addition to the effectiveness of single-drug, IN10018 is expected to overcome the tumor matrix fibrosis barrier and improve the local immune function, so as to improve the efficacy of targeted therapy, chemotherapy and immunotherapy. In August 2021, IN10018 was granted a fast track designation by the FDA for treating platinum-resistant ovarian cancer. In February 2022, the drug was included in the categories of breakthrough therapy designation by CDE for combining with PLD to treat platinum-resistant recurrent ovarian cancer.
The latest Phase 1b study of IN10018 in the treatment of platinum-resistant ovarian cancer shows that: By May 31, 2022, 50 cases with platinum-resistant recurrent ovarian cancer were enrolled in the study, 42 of whom had at least one post-baseline imaging evaluation and were included in the efficacy-evaluable population. Among the 42 efficacy-evaluable patients, 23 patients were partial response (PR), with an ORR of 54.8%; 14 patients were SD, with a DCR of 88.1%. According to PFS analysis, the median PFS of IN10018 combined with PLD in the treatment of platinum-resistant recurrent ovarian cancer reached 7.26 months.
HP530S, a highly-active and highly selective FAK inhibitor, self-developed by the Hinova Pharma, can inhibit metastasis, proliferation and angiogenesis of tumor cells by inhibiting FAK and adjusting its downstream signaling pathway. Preclinical toxicological data shows that the safety of HP530S is controllable. Pharmacodynamics results show that its single-drug can inhibit the proliferation and migration of tumor cells, and its combination therapy has a potent anti-tumor effect. According to the preclinical data: HP530S can inhibit the tumor metastasis and improve local immune function to enhance the efficacy of targeted drugs, chemotherapy drugs and immunotherapy, with a potential to treat solid tumors in combination with various drugs. In February 2023, the IND of HP530S tablet intended for solid tumors was accepted by CDE.
Since the first FAK inhibitor entered the clinic in 2008, the development of FAK inhibitors seems to be in the right way gradually in recent years, which drug combination brings a new life. Although with a slow progress, many products under research have achieved good results in preclinical or clinical trials, which is expected to bring new options for treating platinum-resistant ovarian cancer, pancreatic cancer and other tumors. It is particularly worth mentioning that China's InxMed persistence in the FAK field has brought a new turn for IN-10018.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


