Shruti TalashiOctober 09, 2023
Tag: Continuous Manufacturing , CM process , Unit Operation CM , Batch manufacturing
Over the previous 50 years, pharmaceutical production unit has been using the batch manufacturing concept because of its low setup cost and flexibility. The next batch cannot begin until the previous batch has been done and this process does requires human intervention that is prone to error. Today the batch manufacturing is losing momentum. The world is moving towards automation therefore the change that is being adopted by the manufacturers of drugs is the approach of continuous manufacturing (CM) whereby the material input and output happens simultaneously and throughout. This implies that there won't be any breaks or human intervention in-between the continuous manufacturing process. Apart from the pharmaceutical industry, this process been more prevalent in food/beverage industries as well as chemical industries. This concept of CM was first introduced in the early 19th century by Oliver Evans who developed a continuous flour mill based on this concept. By 1990, The US Food and Drug Administration (FDA) begins to encourage the development and adoption of continuous manufacturing in the pharmaceutical industry. [1]
CM has become the norm, however, pharmaceutical continuous production is still gaining ground. For the treatment of cystic fibrosis, Vertex's Orkambi (lumacaftor/ivacaftor) received the first approval using CM in 2015. The first medication that the FDA permitted to convert from batch manufacturing to continuous manufacture (CM) was Janssen's Prezista (darunavir), used to treat HIV. Six CM-based treatments for diseases such leukaemia, cystic fibrosis, HIV-1 infections, and breast cancer had received FDA approval by the beginning of 2019. The government authorised three applications using CM processes in 2020, including the first regulatory application using CM for API and the first continuous biomanufacturing process, according to Center for Drug Evaluation and Research CDER's 2020 Annual Quality Report. [2]
Increased productivity and efficiency: As there is no downtime between batches and need for human interventions time to time, hence continuous manufacturing can result in significant increase in productivity as well as efficiency.
Cost savings: Continuous production can save costs by eliminating waste and maximising resource efficiency. Nevertheless the technology involved in CM is costly but it will reduce the overall cost of expenditure when compared to batch manufacturing in the long run.
Quality by Design: Product quality can be enhanced with the concept of quality by design (QbD) in CM, which gives manufacturers more control over the production process.
Reduced environmental impact: By consuming less trash and energy, continuous manufacturing can help to lessen the environmental impact of manufacturing and is considered as sustainable production than batch manufacturing.
There are different mode of CM, details regarding this is provided in the ICH Q13 guideline for implementation of CM in the already existing pharmaceutical set up or setting up for new pharmaceutical and bio manufacturing units.
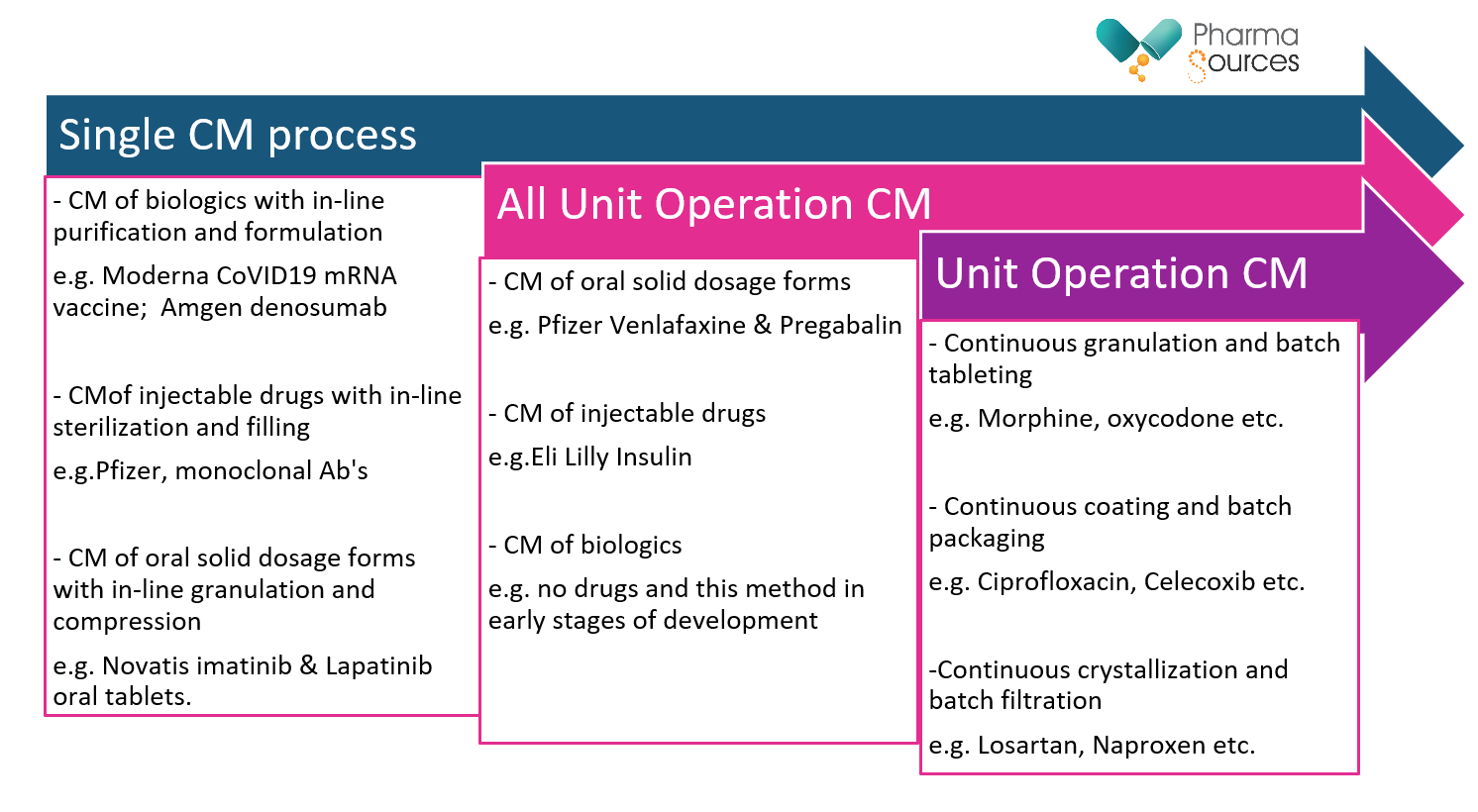
Figure above is showing the three mode of CM as per the ICH Q13 guidelines including different processes with examples of the products that are been manufactured.
1. The considerations listed in Section 3 of the ICH Q13 Guideline ICH Q5E on Continuous Manufacturing of Drug Substances and Drug Products must be taken into consideration when developing a new control strategy for the continuous manufacturing process.
2. Both batch and continuous process output materials must be of a similar calibre & quality. To determine product comparability and determine whether more bioequivalence, non-clinical or clinical investigations, and stability data are required, a scientific and risk-based approach should be adopted.
3. Before changing a batch process that has received regulatory approval into a CM process, manufacturers should do so. Manufacturers can consult the regulatory body for guidance on the regulatory requirements and the acceptability of their approach and data package for the suggested adjustments.
The control strategy for continuous manufacturing processes must be able to identify and address any process deviations in real time. Compared to batch manufacturing procedures, where variations can be found and fixed more quickly, this is more difficult. Through a combination of analytical testing and clinical research, it is possible to establish the product comparability of batch to continuous manufacturing. Any modification to a drug product's manufacturing process requires regulatory approval. Manufacturers should seek regulatory approval before converting an approved batch process to a CM process. Control strategy and product comparability data towards such regulatory approval will make sure the modification won't compromise the drug product's quality or safety. [3]
The development of a successful control strategy for CM is enabled by a holistic approach, considering aspects specific to CM which is Quality by Design QbD & Process analytical technology PAT and the principles are described in ICH Q8– Q11. For instance, the concept QbD has been recently introduced to support pharmaceutical businesses' attempts to achieve market and operational excellence and it was first introduced in the broad field of Quality Management. Currently, QbD is considered to be a crucial enabler for achieving the needed performance quantum leap. Process analytical testing (PAT), also known as process monitoring and control, helps to maintain a state of control during production and enables real-time evaluation of system performance. Process analytical technology (PAT) (ICH Q8) is by nature a good fit for CM. Examples of applications include in-line near-infrared spectroscopy to evaluate blend uniformity, in-line UV flow cells to track therapeutic protein concentration data, and in-line particle size analysis to track crystallizer output. PAT use makes it possible to identify disruptions in real time, therefore proper FDA regulatory counselling & right selection of PAT for a product’s continuous manufacturing is essential. [4]
The factors to assess the process of shifting from batch to continuous manufacturing are:
(a) Technical feasibility- Is it technically feasible to convert the batch into continuous manufacturing by looking at the complexity of the process, nature of the product & availability of the technology;
(b) Regulatory Compliance - A careful consideration of the ICH Q13 Guideline Q5E and other relevant guidance documents; Suggestion, it is always beneficial to get the counselling session with the regulatory bodies.
(c) Cost – Of course, Is the cost of converting to continuous manufacturing justified by the potential benefits?
(d) Risk – For the company, what are the risks associated with converting to continuous manufacturing? Consider, potential for product quality problems, regulatory delays, and any increased costs.
Initially, make a thorough plan for the conversion procedure. Timelines, budgets, and risk mitigation techniques should all be included in this plan. To evaluate the suggested continuous production process and produce data to support the regulatory submission, conduct a pilot study. To switch to continuous manufacturing, submit a regulatory application. Implement the continuous production process and pay close attention to how it is performing. An ongoing process involves evaluating the switch from batch to continuous manufacturing. To guarantee that the level of product quality is maintained, it is crucial to keep an eye on how the continuous manufacturing process is performing and make adjustments as necessary. [5]
Pharmaceutical businesses should think about the advantages and difficulties of adopting continuous manufacturing before deciding if they can do so. An efficient shift to continuous production can be achieved by developing goods with reliable and scalable chemistry, having a methodical process design and efficient process analytical technologies, and utilising a professional workforce with established partnerships to reduce cost risks. Continuous manufacturing is becoming the new norm for pharmaceutical businesses as a result of the expansion of fast-tracked drug production, shorter clinical development pathways to FDA approval, and FDA encouragement. [1,4]
1. IRENE BIRBECK (2020, December 07) Exploring Continuous Pharmaceutical Manufacturing vs. Batch Processing. Exploring Continuous Pharmaceutical Manufacturing vs. Batch Processing (clarkstonconsulting.com)
2. Lowell Zeta, Jim Johnson, Daniel Roberts, and Stephanie Slater (2021, August 23) FDA leads global work on continuous manufacturing approaches to up quality, supply chain resilience Long-awaited ICH Q13 draft guidelines on CM of drug products released. FDA leads global work on continuous manufacturing approaches to up qu - Hogan Lovells Engage
3. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. “ICH Harmonised Tripartite Guideline Q13: Continuous Manufacturing of Drug Substances and Drug Products.” Published July 2021. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q13-continuous-manufacturing-drug-substances-drug-products-step-5_en.pdf
4. Grangeia HB et.al Quality by design in pharmaceutical manufacturing: A systematic review of current status, challenges and future perspectives. European Journal of Pharmaceutics and Biopharmaceutics Volume 147, February 2020, Pages 19-37. https://doi.org/10.1016/j.ejpb.2019.12.007
5. Tom Blackwood (2020, October 07) 6 Key Factors — Should You Go Batch or Continuous? The choice isn’t always clear for particulate solids. 6 Key Factors — Should You Go Batch or Continuous? | Chemical Processing
Ms. Shruti Talashi boasts a dual mastery of lab research and writing. Her doctoral study outcome as M.Phil in biomedical science while studying breast cancer and an extraordinary masters degrees dissertation work on exploring role of Gal-lectin in cancer metastasis fuels her extensive research interests. She has gained few publication in journals. Bridging the science-public gap is her passion, aided by expertise in diverse techniques. From oncology to antibiotic/drugs production, she's led and managed complex projects, even clinical trials. Now, as a freelance Content Coordinator for Sinoexpo Pharmasource.com, her industry knowledge shines through valuable insights on cutting-edge topics like GMP, QbD, and biofoundry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


