PharmaSources/Eric SunMarch 10, 2022
Tag: NCE drug , NME drug , Method Development , Validation Example
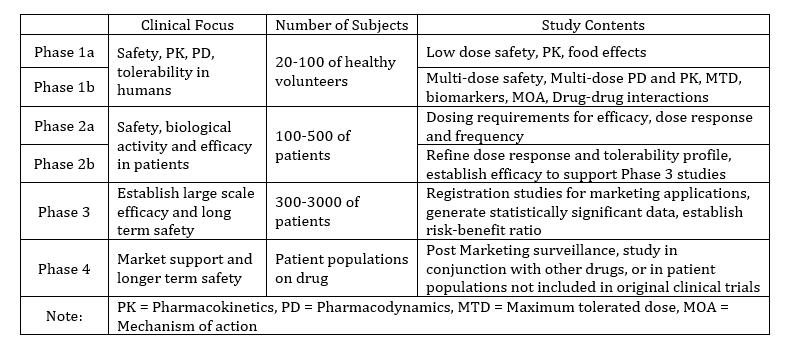
Once a new chemical entity (NCE)/new molecular entity (NME) has been discovered, it is the ultimate goal for all biopharmaceutical companies to develop and deliver the targeted medicine to the patients with the optimum timescale and minimum risk. Compared with a generic drug development, the development process of a NCE/NME drug is much more time consuming and costly. It is estimated that it takes more than 13 years from discovery to approval of a new drug. A 2014 Chemical & Engineering News (C&EN) article reported “Costs to develop new pharmaceutical drug now exceed $2.5 billion” [Ref. 1]. Moreover, the R&D cost is projected to reach $16 billion on single drug development in the year 2043 [Ref. 2]. The lengthy and expensive development process of a NCE drug is typically characterized by the phases of pre-clinical and clinical studies conducted on the drug candidates from Phases 1 to 3, and the post-approval phase (Phase 4). In the early phases (Phase 1 to Phase 2a), the drug development is more frequently subject to the changes involved with the process scale up and optimization, in-process adjustments, altered stability studies with added/deleted container-closure systems, storage conditions or time points, and the continuous evolution of specifications and analytical methods. To the contrary of the late phases (Phases 2b to 3/4 and commercial manufacture), the CMC activities in the early phases of development are not specified by ICH guidelines. As a result, the drug development and its enabling analytical supports and quality control should adopt a phase appropriate and consistent approach to fully take the advantage of regulatory flexibility and to fulfill both the efficiency including the cost saving and the quality expectations. Table 1 briefly summarizes clinical focus, sample size, and study contents at different phases of clinical development.
Table 1. The design and development of clinical trials of drug candidates
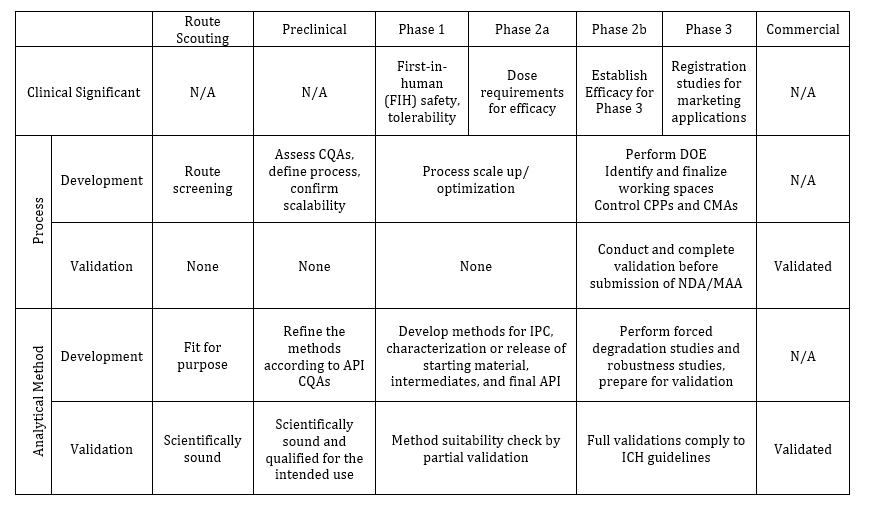
The understanding and practices of phase appropriate approach can vary among companies, at different stages, and can be influenced by funding and business and regulatory environments. For a large, well-established biopharmaceutical company, its R&D department is willing and able to afford ‘nice-to-have’ studies and thorough investigations at early stage to pave a solid foundation for a robust drug development. However, for a small biotech or virtual company with limited funding, the R&D resources must be allocated precisely for the ‘have-to-have’ studies to reach milestones and to get rewarded with infusion of additional funds. In general, phase-appropriate is about what’s needed and when it is needed for each phase to ensure the successful deliveries on a cost-effective basis. To what extent, at what steps, and how much are supposed to be done in the early phases of drug development is up to the decisions of drug developers, which certainly can’t be exercised in the same manner among the large and small companies. However, R&D organizations should not use ‘phase-appropriate’ as an excuse to do whatever is convenient or preferred without limits and boundaries. For example, a Contract Research Organization (CRO) or Contract Development Manufacture Organization (CDMO) should establish internal guidelines to define what R&D works need to be done methodically and consistently at each phase to meet global and regional regulatory requirements; the contracted R&D activities devoted to a project should be science- and risk-based and suitable for the particular development stage, which should not be dictated by the availability of manpower, resources, client budgets or what profits those works will bring in. By the same token, an individual pharmaceutical company should take a ‘consistent phase-based’ approach over the course of drug development: consistently perform necessary actions in a structured and predictive manner from project to project within the scopes of GLP or GMP requirements suitable for each specific phase. Table 2 is employed here to illustrate phase-based development and validation activities across product lifecycle. Table 2 binds no parties on how to perform consistent phase-based development and validation, but rather serves to stimulate streamlined discussions toward consensus. In addition, HPLC assay and impurity method development and validation is used as an example in this article to demonstrate the proposed consistent phase-based analytical supports.
Table 2. Phase-based Process and Analytical Developments and Validations at Different Phases


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


