Yi/PharmaSourcesMay 29, 2023
Tag: Roxadustat , generic drug , CKD-related anemia
On May 22, Nanjing Chiatai Tianqing Pharmaceutical Co., Ltd. submitted a Class 4 generic drug marketing application for Roxadustat Capsules that was accepted by the CDE, which means the first generic Roxadustat drug was reported for production. In May 2023, the CDE announced the acceptance of eight generic drug marketing applications for Roxadustat from: Nanjing Chiatai Tianqing, Wanbangde Pharmaceutical, Shanxiang Pharmaceutical, Chengdu Brilliant.
Roxadustat is the first small molecule hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF- PHI) to be developed worldwide. The physiological effects of hypoxia-inducible factor (HIF) increase the expression not only of erythropoietin but also of erythropoietin receptors and proteins that promote iron uptake and circulation. Roxadustat inhibits prolyl hydroxylase (PH) enzyme by mimicking ketoglutarate, one of the substrates of PH, and affects the role of PH enzyme in maintaining the balance between the rate of HIF production and degradation for the purpose of correcting anemia.
The original Roxadustat was discovered by FibroGen and developed in collaboration with the Japanese pharmaceutical company Astellas Pharma Inc. In addition, FibroGen has partnered with AstraZeneca on the co-development and commercialization of Roxadustat in the U.S., China, and other markets in the Americas, Australia/New Zealand, and Southeast Asia.
China is the first country to approve Roxadustat. In December 2018, the NMPA approved Roxadustat capsules through priority drug review and approval (brand name: Aizhuorui) for the treatment of anemia in CKD dialysis patients through a priority review approval process. In August 2019, the NMPA approved an expanded new indication for Roxadustat Capsules for the treatment of non-dialysis-dependent CKD anemia. In November 2019, Roxadustat is included in the scope of the national health insurance through national health insurance negotiations. In November 2020, the first Clinical Practice Guidelines for the Treatment of Renal Anemia in China was officially released, and HIF- PHI represented by Roxadustat entered the guidelines for the first time and was recommended with the highest intensity.
In addition, in May of this year, a randomized, open, positive-controlled, multicenter phase 3 study evaluating the efficacy and safety of Roxadustat for the treatment of anemia in subjects with non-myeloid malignant tumors undergoing chemotherapy in China was successful; Roxadustat demonstrated non-inferiority compared to recombinant human erythropoietin alpha (SEPO®) on the primary endpoint of mean change from baseline in mean Hb levels at weeks 9-13.
However, in the same month, MATTERHORN, a phase 3 study of Roxadustat for the treatment of transfusion-dependent low-risk myelodysplastic syndrome (MDS) patients with anemia, failed miserably, failing to meet the primary efficacy endpoint; The proportion of patients achieving red blood cell transfusion independence (RBC-TI) in the first 28 weeks was 47.5% in the Roxadustat group compared to 33.3% in the placebo group (P = 0.217), with no statistically significant difference.
Up to now, Roxadustat has been approved for marketing for the treatment of CKD-related anemia in Japan, Chile, Korea, and the European Union. However, Roxadustat's marketing application in the U.S. was tragically delayed twice and ultimately denied.
With the introduction of health insurance, the sales volume of Roxadustat has climbed year after year. Net sales volume of Roxadustat in China for FibroGen and AstraZeneca are reported to be $208.8 million (approximately RMB 1.46 billion) in 2022, compared to $186.1 million for the full year 2021, representing a 12% increase in net sales volume and more than 80% increase in sales.
It is reported that the patent for the Roxadustat compound partially expires in 2024, the patent for crystal form expires in 2033 and the patent for preparation expires in 2034. At present, more than 10 enterprises in China are laying out the generic drug market for Roxadustat and conducting bioequivalence tests, as detailed in the table below.
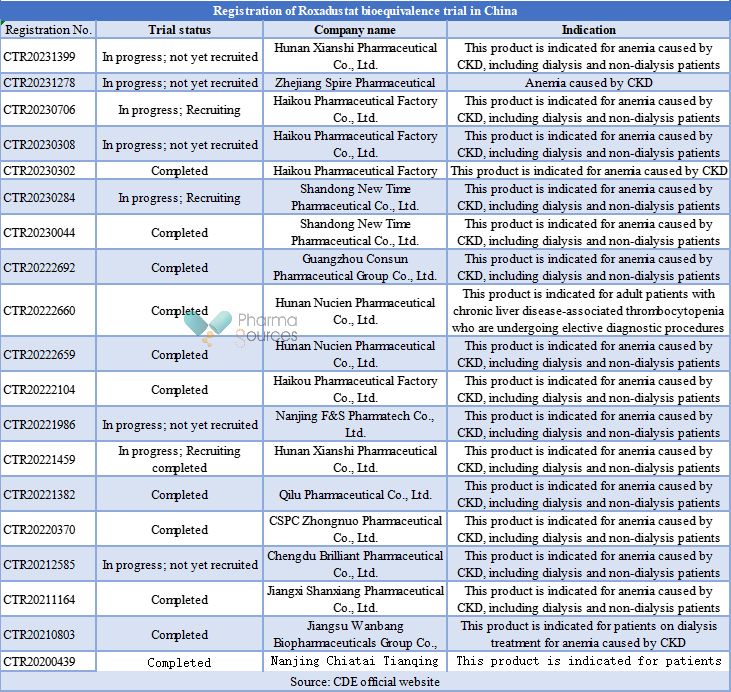
Nanjing Chiatai Tianqing Pharmaceutical Co., Ltd. is the first enterprise to register a Roxadustat trial of biological and other news, and is expected to be the first to submit a generic drug version of Rosasta for marketing, and is expected to be the first to obtain a generic drug manufacturing approval for Roxadustat. However, it is not clear who will win the generic drug competition for Roxadustat in the future, after all, several enterprises have already completed Roxadustat bioequivalence trials.
In addition, it is worth mentioning that the indications for the Roxadustat bioequivalence trial are mainly CKD-related anemia. However, Hunan Nucien PharmaceuticalCo., Ltd. also conducted a bioequivalence trial for the indication of chronic liver disease-associated thrombocytopenia for elective diagnostic operations or surgery.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


