PharmaSources/YefenghongAugust 02, 2021
Tag: Rec-Biotechnology , HPV , IPO
On July 16, Rec-Biotechnology submitted its prospectus to HKEX and was accepted. Rec-Biotechnology is an innovative vaccine company, and its core product, Human Papillomavirus 9-valent Vaccine, Recombinant, is in Phase III clinical trial stage. According to the prospectus, the co-sponsors of this IPO are Morgan Stanley, CMB International and CITIC Securities, and the funds raised will be mainly used for the research and development of vaccine.
Rec-Biotechnology has developed a comprehensive vaccine innovation engine, including a new adjuvant platform, a protein engineering platform and an immune evaluation platform. The company is one of the few companies capable of developing new adjuvants, and is able to benchmark all new adjuvants currently approved by the FDA. The company's technology platform has formed an "iron triangle", which has created synergistic effects in antigen design and optimization, adjuvant development and production, and determination of the best combination of antigen and adjuvant.
The company has established a product pipeline including 11 innovative vaccines under research, which are used to treat diseases such as cervical cancer, COVID-19, adult tuberculosis, herpes zoster, hand-foot-mouth disease and influenza. However, the core product is REC603, a Human Papillomavirus 9-valent Vaccine, Recombinant, which is currently in Phase III clinical trial stage and is also the company's vaccine closest to commercialization. Let's take a closer look.
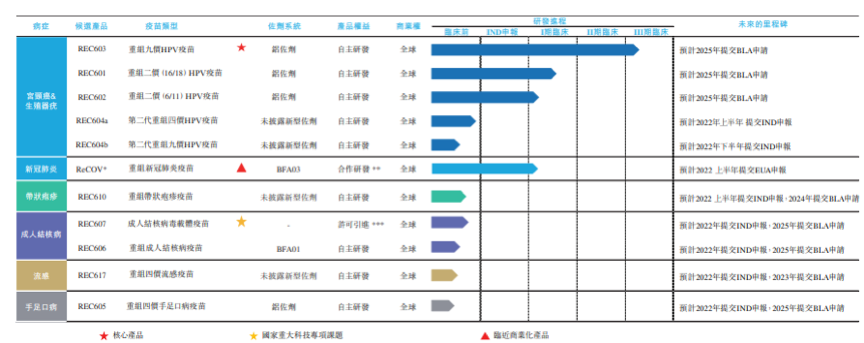
Research and Development Pipeline of Rec-Biotechnology (Source: Rec-Biotechnology Prospectus)
REC603 is a Human Papillomavirus 9-valent Vaccine, Recombinant, which aims to provide protection against HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58. REC603 adopts hansenula expression system, and the methanol utilization pathway of hansenula has strong and adjustable promoter, high secretion, low glycosylation level and other characteristics, which is suitable for the production of medical recombinant protein.
The Phase I clinical trial of REC603 has proved that it has good immunogenicity and safety. At present, the product is in the Phase III clinical trial stage, and it is expected to submit BLA to the State Food and Drug Administration in 2025. In addition to REC603, Human Papillomavirus 2-valent Vaccine, Recombinant candidate vaccines developed by the company -- REC601 and REC602 for HPV 16/18 and HPV 6/11 respectively, have entered Phase I clinical trials.
As far as HPV market is concerned, it has great potential. Since the first HPV vaccine has been approved in 2017, the market volume has increased to RMB 13.1 billion in 2020, and it is expected to grow to RMB 69 billion in 2030 at a compound annual growth rate of 18%. At present, among the four HPV vaccines listed in China, the only approved Human Papillomavirus 9-valent Vaccine, Recombinant, has a market share of over 48.5%.
In addition to HPV vaccine, COVID-19 vaccine (ReCOV), a product of Rec-Biotechnology, is one of the most concerned products at present, and is undergoing Phase I clinical trial in New Zealand. It is worth mentioning that the actual research and development of this project is carried out by Jiangsu Provincial Center for Disease Control and Prevention and Taizhou Medical New & High-tech Industry Development Zone, while Rec-Biotechnology is responsible for providing funds for research and development of ReCOV and providing several technical platforms in the whole development cycle.
The research and development team adopted a new vaccine design concept based on antigen target guided by neutralizing antibody. After analyzing the binding sites and mechanism of RBD and NTD, two key domains of novel coronavirus Spike (S) protein, and their corresponding humanized neutralizing antibodies FC08 and FC05, the concept of neutralizing antibody "Cocktail" was put forward. In addition, the concept of the next generation genetic engineering vaccine of SARS-CoV-2 aiming at RBD and NTD subunits of S protein was designed and functional verification was completed. If you are looking for list of bioproducts, then please come to Pharmasources.
The RBD and NTD dual target selected by ReCov is an important innovation, and its advantages have been verified:
1) It contains key neutralization site areas and reduces non-neutralization site areas, so it has better immunogenicity and low adverse reactions;
2) The large-scale production process is simple, the output is high, the production capacity can be quickly enlarged, and the manufacturing cost is low;
3) The vaccine has good stability, which can be stable at room temperature for more than three months, and can be widely inoculated in underdeveloped countries and tropical regions.
In addition to HPV and COVID-19 vaccines, Rec-Biotechnology's product pipeline also includes recombinant herpes zoster candidate vaccine, adult tuberculosis candidate vaccine, four-strain flu vaccine (recombinant) candidate vaccine, hand-foot-mouth disease 4-valent vaccine (recombinant) and so on.
According to Frost & Sullivan, innovative vaccines will account for about 55.4% of China's vaccine market in 2020, and this proportion is expected to further increase to 86.6% in 2030. Driven by the ever-increasing innovative vaccines and technological breakthroughs, the volume of China's vaccine market is expected to increase from RMB 75.3 billion in 2020 to RMB 333.3 billion in 2030, with a compound annual growth rate of 16.0%.
As many products of Rec-Biotechnology are still in the research and development and clinical stage, a lot of money will be needed for a long time. During this period, in addition to facing the risk of research and development failure, the company's products will also face the dilemma of intensified competition. Compared with its competitors, Rec-Biotechnology' research and development of HPV and COVID-19 vaccine are relatively backward, and will face fierce competition after being approved for listing.
1. Rec-Biotechnology Prospectus;
2.Retrieved July 18,2021, from https://www1.hkexnews.hk/app/sehk/2021/103663/documents/sehk21071601366_c.pdf.
Ye Fenghong, a medical editor specializing in oncology at a healthcare internet company, has conducted in-depth research on the pathogenesis and clinical treatment of lung cancer and breast cancer. She has previously been involved in the design and synthesis of anti-tumor drugs and has some experience in computer-aided drug design. She is currently devoted to introducing cutting-edge cancer treatment drugs to a wide range of readers, aiming to help more people avoid cancer pain and embrace good health.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


