PharmaSources/DopineJuly 26, 2021
Tag: BTK Inhibitor , Orelabrutinib , InnoCare , Biogen
On July 13, InnoCare Pharma and Biogen made joint announcement that they have entered into an agreement on the license and cooperation of the BTK inhibitor Orelabrutinib.
As per the agreement, Biogen will claim the exclusive global rights of Orelabrutinib in the field of multiple sclerosis, plus the exclusive rights of Orelabrutinib in certain autoimmune diseases in regions outside China (including Hong Kong, S.A.R., China, Macau, S.A.R., China and Taiwan, China). InnoCare Pharma retains the exclusive global rights of Orelabrutinib in the field of oncology and the exclusive rights of certain autoimmune diseases in China (including Hong Kong, S.A.R., China, Macau, S.A.R., China and Taiwan, China). InnoCare Pharma will garner a down payment of 125 million US dollars and be eligible to gain up to 812.5 million US dollars at potential clinical development milestones and commercial milestone payments when the those milestones and sales milestones are met.
Orelabrutinib is known to be a class 1 innovative drug independently developed by InnoCare Pharma. It features a new type of BTK inhibitor that has a high target selectivity in the treatment of lymphoma and autoimmune diseases. In December 2020, the drug was issued with a license from NMPA for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), as well as two indications of R/R MCL.
BTK is recognized as a key kinase in the B cell receptor signaling pathway. It plays a vital role in the development and function of immune cells, such as B lymphocytes, macrophages and microglia, involved in the pathological process of MS. It is hopeful that BTK inhibitors will make available novel treatment options for the treatment of autoimmune diseases including MS.
It has been reported in studies that Orelabrutinib in the field of multiple sclerosis is identified to have high selectivity and blood-brain barrier permeability, which allows for a high target occupancy rate in the central nervous system. At present, Orelabrutinib is undergoing a global phase II clinical study for patients that experience relapsing-remitting multiple sclerosis (RRMS). Furthermore, Orelabrutinib has also been rolled out to Phase IIa clinical trials for systemic lupus erythematosus (SLE) in China.
Since CHIPSCREEN's Chidamide was available overseas in 2007, sorts of new drugs have been granted with an overseas license. Incomplete statistics reveal that, so far, at least 17 overseas licenses have been released in 2021. The transaction products chiefly fall into tumor and autoimmune disease drugs, with 3 transactions topping 1 billion US dollars.
License-out Projects in 2021
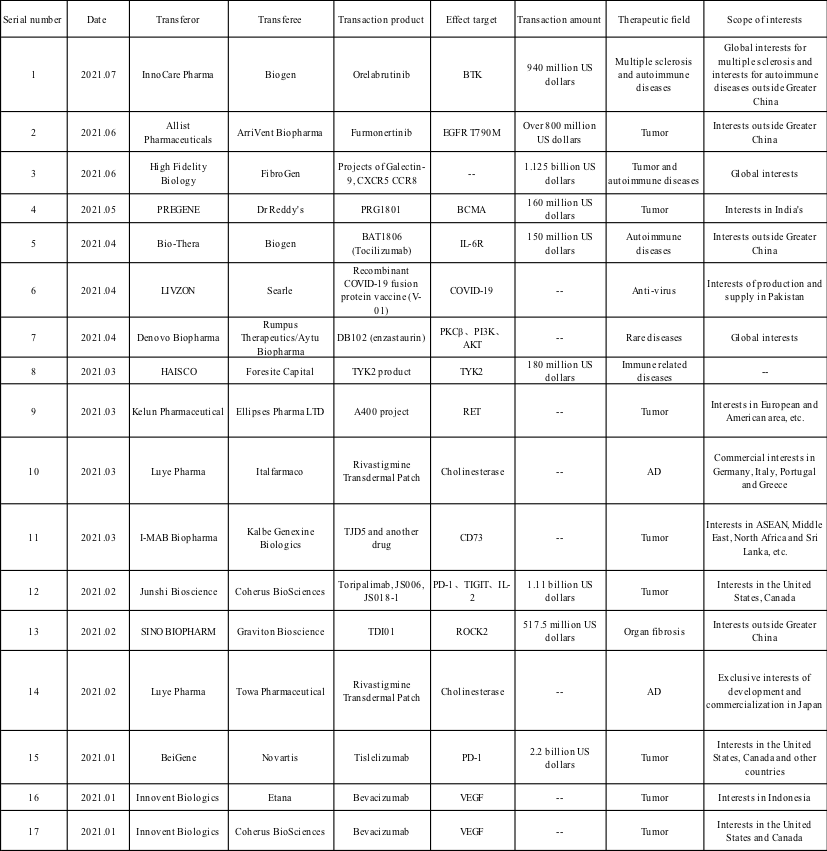
Orelabrutinib is established as the second domestically authorized BTK inhibitor in China.
Furmonertinib is deemed as a third-generation EGFR-TKI developed by Allist Pharmaceuticals. It was cleared in China in March 2021 for application in preventing disease progression during and after treatment with EGFR-TKI, and for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with confirmed positive EGFR T790M mutation. Its trade name is Ivesa.
PRG1801 is perceived as a targeted BCMA CAR-T cell injection product independently developed by PREGENE, who owns global independent intellectual property rights. Its clinical research in China has treated 34 patients with relapsed and refractory multiple myeloma, showing remarkable efficacy and breathtaking safety.
BAT1806 is known to be a Tocilizumab injection developed by Bio-Thera in line with applicable guidelines of China's NMPA, US FDA, and EU EMA bio pharma products. As a recombinant humanized monoclonal antibody targeting IL-6R, it is currently in the global Phase III clinical trial.
DB102 is identified as the first small molecule serine/threonine kinase inhibitor worldwide. Acting on key tumor targets in the field of tumors such as PKCβ, PI3K and AKT, it presents direct effects of inducing tumor cell death and hindering tumor cell proliferation, as well as indirect effects of inhibiting tumor-induced angiogenesis. It was in the first place developed by Eli Lilly for the treatment of newly-treated high-risk diffuse large B-cell lymphoma (DLBCL) and glioblastoma (GBM). The transaction will authorize the interests of DB102 (Enzastaurin) to treat rare genetic diseases vEDS to Aytu BioPharma.
Rivastigmine is designed as a cholinesterase inhibitor drug for the treatment of mild to moderate dementia related to AD.
TJD5, (Uliledlimab) a highly differentiated CD73 humanized monoclonal antibody, is independently developed by I-MAB Biopharma. It fully inhibits the activity of CD73 with the binding mode of "intra-dimer" by recognizing unique epitopes, thereby achieving superior anti-tumor effect.
TDI01 is an oral small molecule drug based on a new target and a new mechanism independently developed by Beijing Tide Pharmaceutical. As a highly selective Rho/Rho-related coiled-coil protein kinase 2 (ROCK2) inhibitor, its current clinical trial indication is directed to lung fibrosis, non-alcoholic steatohepatitis, etc.
These products for transaction lie in the clinical trial stage except for Furmonertinib, Orelabrutinib, Toripalimab, Tislelizumab and Rivastigmine Transdermal Patch. These products under development come with novel targets and less competition of the same kind. From the perspective of drug types, the number of antibody drugs and small molecule drugs in the trading products is not bad.
It is noteworthy that the 4 PD-1 mAbs (Camrelizumab, Toripalimab, Tislelizumab and Sintilimab) approved for marketing in China, and the two domestically produced third-generation EGFR-TKI inhibitors (Almonertinib and Furmonertinib) have gone international.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


