Neeta RatanghayraAugust 06, 2021
Tag: Alzheimer's Disease , Biogen , aducanumab
Alzheimer's disease (AD) is a neurodegenerative disease affecting millions of people globally. AD is a chronic, progressive disease and with time, the patient’s cognition always deteriorates.
Apart from the typical symptoms of memory loss and cognitive impairment, AD is associated with a long prodromal period of 20 to 30 years during which no symptoms are seen. By the time the disease is diagnosed, significant, irreparable damages may have occurred.
The current treatment options offer symptomatic relief, but there is a huge unmet need for treatment with disease-modifying activity. The recent approval of Biogen’s aducanumab marks the beginning of a pipeline of therapies meant to target the underlying disease mechanism - but “one size may not fit all”. Hence, finding alternative treatment options and biological pharma products especially those which target the disease pathophysiology is the need of the hour.
A distinct pathological feature of AD is the accumulation of amyloid β plaques in the brain which triggers neurodegenerative processes leading to memory loss and cognitive impairment. Another hallmark is the presence of tau protein tangles. Treatments targeting amyloid β plaques and phosphorylated tau protein have been investigated in several clinical trials albeit with limited success. This has compelled research towards targeting other targets such as inflammation, oxidative stress, infection, metabolism/bioenergetics, vascular factors, synaptic plasticity/neuroprotection, and gut-brain axis.
Currently, there are around 126 drug candidates for AD at various phases of clinical development. Of these, 28 agents are being tested in phase 3 trials – these include symptomatic agents as well as disease-modifying treatments. The list also includes 10 repurposed agents.
Some of the promising AD pipeline disease-modifying treatments in phase III clinical trials
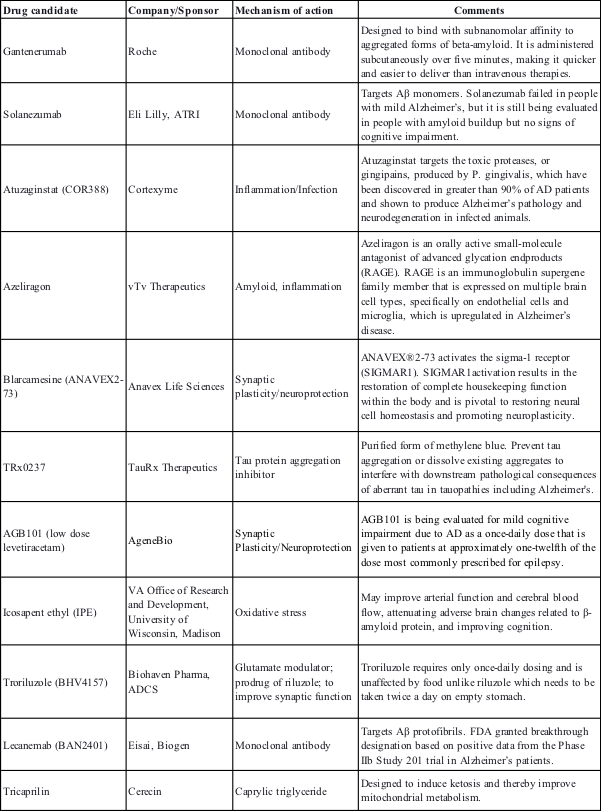
In the past few years, several treatments targeting various AD mechanisms especially amyloid β have been designed and tested, however, most have a disappointing track record.
Several reasons have been postulated for clinical trial failure. Some experts suggest that the dose used in the trials could be inadequate to achieve the therapeutic effect. Due to the differences in the blood-brain barrier between humans and animals, the dose used on animal models to predict human blood-brain barrier drug permeability may be insufficient to elicit a response in humans. Also, the doses used may result in unacceptable adverse effects. Several trials were stopped early due to inflammatory changes or microhemorrhages in the brain.
Another debate lingering AD trials is targeting the disease stage at which it is reversible. Most trials that failed target mild to moderate disease. A preclinical phase that precedes the onset of symptoms by a couple of decades is seen as a potentially reversible stage.
Though amyloid and tau are seen as potential targets, the stream of failed clinical trials indicates that other potential targets need to be explored. Also, research shows that amyloid β and tau protein act together synergistically in AD, thus, a possible approach would be to target both amyloid β and tau simultaneously.
On June 07, 2021, the US FDA approved Biogen’s aducanumab (Aduhelm) for the treatment of AD.
Aducanumab is a monoclonal antibody that targets the amyloid-β and reduces amyloid plaques in the brain. Aducanumab is the first AD therapy approved to target an underlying disease mechanism.
The FDA approved aducanumab using the accelerated approval pathway based on the surrogate endpoint of reduction of amyloid-beta plaque in the brain. Aducanumab was evaluated in three different studies including a total of 3,482 patients with AD. Compared to placebo, in patients receiving aducanumab, a significant reduction of amyloid-beta plaque was observed.
FDA’s accelerated approval pathway allows the earlier approval of drugs that treat serious conditions, and that fill an unmet medical need based on a surrogate endpoint. The use of a surrogate endpoint significantly shortens the time required before receiving approval. Under the accelerated approval pathway, Biogen is required to verify the aducanumab’s clinical benefit in a new randomized, controlled clinical trial.
Around 50 million people worldwide are afflicted with AD, of this at least 10 million patients are in China. The numbers are expected to touch 40 million in China by 2050. The prevalence of AD is higher in older age groups, among females, and in the rural areas of China. The growing prevalence and high burden call for an increased demand for new drugs for this devastating disease.
In November 2019, the National Medical Products Administration (NMPA) approved GV-971 (sodium oligomannate), the first AD drug to be marketed since 2003.
GV-971 is a mixture of acidic linear oligosaccharides derived from brown algae. The drug is reported to work by modifying gut bacteria. When administered orally, GV-971 is retained in the gut where it reduces bacterial metabolite-driven peripheral infiltration of immune cells into the brain, inhibits Aβ aggregation, and inhibits neuroinflammation in the brain. The drug penetrates the blood-brain barrier through transporters including the glucose transporter GLUT1.
GV-971 completed a nine-month phase III trial in 2018 in China including 818 patients across 34 public hospitals. The drug was found to demonstrate a safe and effective improvement in cognition. In October 2020, a global phase III trial of GV-971 was initiated in around 2,000 patients at 200 medical centers worldwide. The trial is expected to be completed by 2025.
AD accounts for 60–70% of dementia cases, which contributes to increased disability and social dependency. Due to these devastating consequences, more effective therapies are urgently required.
Experts feel that approvals of the first drug in a new category can revive the field, leading to greater innovation and increased investments. Developing new ideas and targeting those in large-scale clinical trials can help in better understanding the treatment effectiveness.
1. Shi J, Sabbagh MN, Vellas B. Alzheimer's disease beyond amyloid: strategies for future therapeutic interventions. BMJ. 2020;371:m3684.
2. FDA Grants Accelerated Approval for Alzheimer’s Drug. Available at: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
3. Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer's disease drug development pipeline: 2021. Alzheimers Dement (N Y). 2021;7(1):e12179.
4. Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. 2020;27(1):18.
5. Wang X, Sun G, Feng T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. 2019;29(10):787-803.
6. Sodium Oligomannate (GV-971). Alzheimer’s Drug Discovery Foundation. Available at: https://www.alzdiscovery.org/uploads/cognitive_vitality_media/Sodium_Oligomannate_(GV-971).pdf
Neeta Ratanghayra is a freelance medical writer, who creates quality medical content for Pharma and healthcare industries. A Master’s degree in Pharmacy and a strong passion for writing made her venture into the world of medical writing. She believes that effective content forms the media through which innovations and developments in pharma/healthcare can be communicated to the world."
This article is first published on
Pharma Sources Insight August 2021



Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


