PharmaSources/DopineAugust 06, 2021
Tag: Furmonertinib , Avitinib , Almonertinib
According to Allist Pharmaceuticals' announcement on June 30, Allist intends to enter into an agreement with ArriVent Biopharma (hereinafter referred to as "ArriVent") to authorize ArriVent the right to exclusively develop (including R&D, production, import, export, use, sales, etc.) Furmonertinib outside Greater China, and Allist will receive a down payment of 40 million US dollars, and an accumulative amount of no more than 765 million US dollars, including R&D and sales milestone payments (after reaching the agreed R&D or sales milestone event), sales commission fees, and some shares of ArriVent.
Furmonertinib is a highly selective and irreversible third-generation EGFR-TKI independently developed by Allist. In March 2021, it was approved for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) in adults with disease progression during or after treatment with EGFR-TKI and positive EGFR T790M mutation confirmed by detection. Furmonertinib's trade name is Ivesa.
Furmonertinib marks the third 3G EGFR-TKI and the second domestic EGFR-TKI approved in China. The Phase 2b clinical data published in The Lancet Respiratory Medicine revealed that Furmonertinib came with an ORR of 74%, mPFS of 9.6 months, and DCR of 94% in the treatment of patients with EGFR T790M mutation-positive locally advanced or advanced NSCLC; In terms with patients with brain metastases, according to the data disclosed in the WCLC 2020, the 160 mg subgroup of the Phase 2a dose extension study suggested Furmonertinib proved central nervous system (CNS) ORR of 84.6 %, CNS mPFS of 19.3 months, and CNS DCR of up to 100%.
Moreover, the production application of Furmonertinib for the first-line treatment of NSCLC (i.e. the treatment of locally advanced or metastatic NSCLC adult patients with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation) are expected to be filed this year. Meanwhile, this drug for adjuvant therapy of the indication (of patients with EGFR mutation-positive stage II-IIIA NSCLC after radical resection with or without adjuvant chemotherapy) is currently subject to Phase 3 clinical trials. Furmonertinib for indications for insertion mutation of exon 20 (of locally advanced or metastatic NSCLC adult patients with EGFR 20 exon insertion mutations) is reported to be in Phase 1b clinical trials.
So far, three 3G EGFR-TKI inhibitors have been permitted in China, details of which are listed in the following table. Therein, AstraZeneca's Osimertinib has been authorized for second-line treatment, first-line treatment, and postoperative adjuvant therapy for patients with EGFR mutation-positive NSCLC, and yet Almonertinib and Alflutinib are only allowed for second-line treatment. Simultaneously, marketing application of Almonertinib for the first-line treatment of new indications of adult EGFR exon 19 deletion or exon 21 (L858R) substitution mutations of locally advanced or metastatic NSCLC (the relevant acceptance number is CXHS2101017) has been brought into the priority review by CDE.
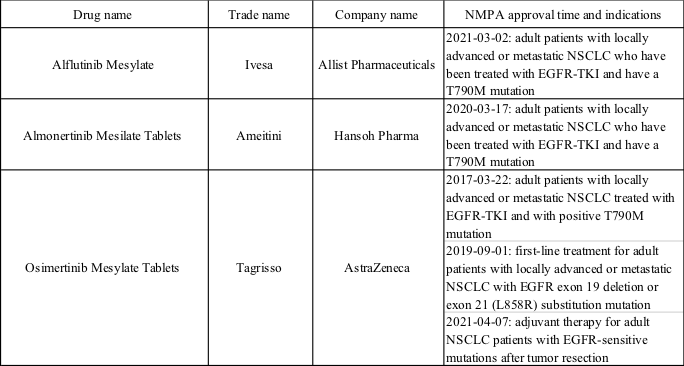
Furthermore, the production application of 4 another third-generation EGFR-TKIs have been submitted, including 4 domestic new drugs and 1 generic drug.
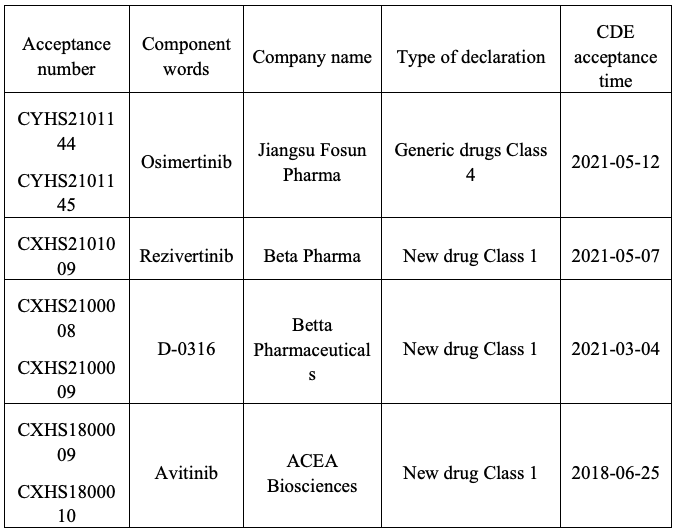
It can be perceived from the approval and production applications of the third-generation EGFR-TKIs that the R&D strength of Chinese pharmaceutical companies shouldn't be belittled, and they have been accredited and favored by overseas pharmaceutical companies. thereinto, Avitinib and Almonertinib have gone global. In May 2020, ACEA Biosciences issued exclusive authorization to Sorrento Therapeutics for all indications of Avitinib in all parts outside China. In August 2020, Hansoh Pharma authorized the overseas development and commercialization rights of Almonertinib (outside Chinese Mainland, Hong Kong, S.A.R., China, Macau, S.A.R., China and Taiwan, China) to EQRx.
Besides the third-generation EGFR-TKIs, incomplete statistics have disclosed a number of drugs and biological pharma products of China have been made available overseas in H1 of this year, including two homemade PD-1 monoclonal antibodies (i.e. Tislelizumab and Toripalimab) and 2 biosimilars (i.e. Bevacizumab biosimilar and Tocilizumab biosimilar).
In January 2021, Innovent Biologics licensed the commercialization rights of Bevacizumab biosimilars in the United States and Canada to Coherus, and then exclusively authorized Etana the rights and interests of such biosimilar in Indonesia.
In January 2021, BeiGene granted the development, production and commercial rights and interests of Tislelizumab in the United States, Canada, Mexico, EU member states, the United Kingdom, Norway, Switzerland, Iceland, Liechtenstein, Russia and Japan=to Novartis.
In February 2021, Tide Pharmaceutical, a subsidiary of CNBG, concluded an overseas licensing cooperation agreement with Graviton to jointly develop and commercialize its self-developed innovative drug TDI01 for the treatment of fibrosis beyond Greater China. TDI01 is known to be a highly selective inhibitor of the new target Rho/Rho-related coiled-coil forming protein kinase 2 (ROCK2), which is able to inhibit the progression of fibrosis, anti-inflammatory and immune regulation by inhibiting the ROCK2 signaling pathway based on high selectivity.
In February 2021, Luye Pharma and Towa Pharmaceutical reached an agreement, which licensed the latter the exclusive right to develop and commercialize Rivastigmine Transdermal Patch in the Japanese market. In March 2021, Luye Pharma arrived to an agreement with Italfarmaco Group, which licensed the latter the exclusive right to commercialize its Rivastigmine Transdermal Patch in the following European countries: Germany, Italy, Portugal and Greece. Based on this agreement, Italfarmaco also has the preferred right to commercialize the product in Chile and Vietnam.
In February 2021, Junshi Bioscience and Coherus Corporation came to a cooperation on the development and commercialization of Toripalimab in the United States and Canada, which allowed Coherus to obtain the license of Junshi Bioscience's Toripalimab and two optional projects (in case of implementation) in the United States and Canada.
In March 2021, I-MAB Biopharma and Kalbe Genexine Biologics (hereinafter referred to as "KG Bio") reached a strategic partnership. The agreement licenses KG Bio the preferred negotiation right for the exclusive authorization of commercialization of two drug candidates independently developed by I-MAB Biopharma, including: TJD5, a highly differentiated anti-CD73 antibody for advanced solid tumors, which has been in the Phase 1 clinical trial; and another drug candidate of I-MAB Biopharma to be agreed between the two parties.
In March 2021, Kelun-Biotech, a subsidiary of Kelun Pharmaceutical, and Ellipses Pharma entered into a regional licensing cooperation agreement, in which Kelun-Biotech authorized the exclusive license of its small-molecule tumor-targeted RET kinase inhibitor project (A400 project) in Europe and the United States and other regions to Ellipses Pharma at a certain price.
In April 2021, Denovo Biopharma delegated the global clinical development and commercialization rights of DB102 to treat rare genetic diseases such as vEDS to Rumpus Therapeutics/Aytu Biopharma. DB102 is regarded as a "first-in-class" small-molecule serine/threonine kinase inhibitor and acts on key tumor targets such as PKCβ, PI3K and AKT. It shows direct effects of inducing tumor cell death and hindering tumor cell proliferation, and the indirect effect of inhibiting tumor-induced angiogenesis. It was originally developed by Eli Lilly for the first-line treatment of high-risk DLBCL and GBM, and Denovo Biopharma currently owns the global rights of such drug.
In April 2021, Bio-Thera licensed BAT1806's product rights in the global market beyond China (including Chinese Mainland, Hong Kong, S.A.R., China Special Administrative Region, Macau, S.A.R., China Special Administrative Region and Taiwan, China) to Biogen. BAT1806 is reported to be a Tocilizumab injection developed by Bio-Thera in accordance with the relevant guidelines of China's NMPA, US FDA, and EU EMA biosimilars. It is established as a recombinant humanized monomer cloned antibody targeting interleukin-6 receptor (IL-6R), and it is in the process of a global Phase III clinical study for the treatment of rheumatoid arthritis.
In May 2021, PREGENE exclusively authorized the clinical development and commercialization rights of its self-developed Nanobody-based CAR-T cell injection product in India to Dr. Reddy's Laboratories.
This article is first published on
Pharma Sources Insight August 2021



Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


