PharmaSources/ChuxinJuly 09, 2021
Tag: Solid Tumor , CARSGEN , Hong Kong IPO , CAR-T
On June 18, CARSGEN Therapeutics, a pioneer in the field of CAR-T cell therapy, officially listed on the Hong Kong, S.A.R., China Stock Exchange, offering 94.747 million shares, priced at HK $32.80 per share, raising a net of about HK $2.94 billion.
Since establishment, CARSGEN Therapeutics has been focused on the research and development of the innovation of CAR-T cell therapy and differentiated innovative medical supplies products. The CAR-T product candidates of CARSGEN are evidence-based novel tumor-associated antigens that have been carefully designed and optimized to reduce the side effects common to existing CAR-T therapies. CARSGEN Therapeutics has independently developed 11 product candidates for the treatment of solid tumors and hematologic malignancies.
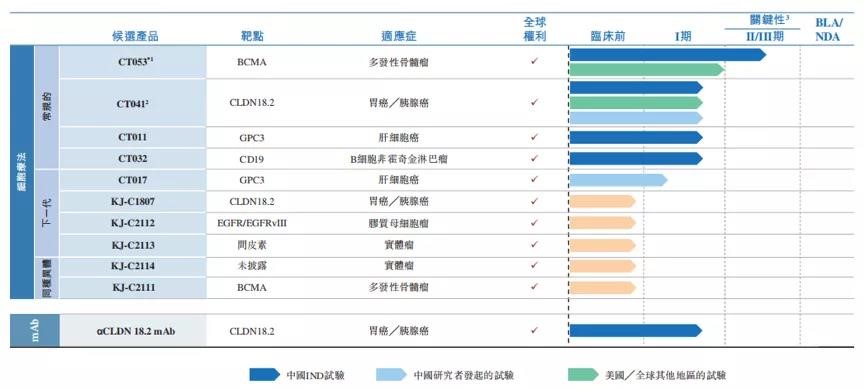
R&D line of CARSGEN Therapeutics (Source: reference 1)
候选产品 | Candidate products |
靶点 | Targets |
适应症 | Indications |
全球权利 | Global rights |
临床前 | Preclinical |
I期 | Phase I |
II/III期 | Phase II/III |
关键性 | Criticality |
多发性骨髓瘤 | Multiple myeloma |
胃癌/胰腺癌 | Gastric cancer/pancreatic cancer |
肝细胞癌 | Hepatocellular carcinoma (HCC) |
B细胞非霍奇金淋巴瘤 | B cell non-Hodgkin's lymphoma |
胶质母细胞瘤 | Glioblastoma |
实体瘤 | Solid tumors |
中国IDN试验 | Chinese IDN trails |
中国研究者发起的试验 | A trial led by Chinese researchers |
美国/全球其他地方的试验 | A trial led by US/other place of the world |
细胞疗法 | Cell therapy |
同种异体 | Allogeneic |
下一代 | Next generation |
常规的 | Conventional |
间皮素 | Mesothelin |
未披露 | Undisclosed |
CT053 is the core product candidate of CARSGEN Therapeutics and the one with the fastest progress in its R&D line. This is an updated candidate of an autologous CAR-T targeting B-cell maturation antigen (BCMA) with a primary target indication of relapsed/refractory multiple myeloma (R/R MM ). BCMA is a member of the tumor necrosis factor receptor (TNF-α) superfamily, which is mainly expressed in mature B lymphocytes and plasma cells. One of its most prominent features is that it is expressed in all MM cells, making it an ideal antigenic target for the treatment of MM.
At the International Multiple Myeloma Symposium 2019 (IMW 2019), CARSGEN Therapeutics announced the clinical trial results of CT053: The objective response rate (ORR) and complete response rate (CR) were 87.5% and 79.2% in R/R MM patients treated with CT053, respectively.
At the annual meeting of the American Society of Hematology (ASH 2020), CARSGEN Therapeutics announced the latest results of CT053 in the treatment of MM. These included the results of Phase I registered clinical trials in China, the 24 months follow-up results of exploratory clinical trials in China, and the results of Phase Ib registered clinical trials in North America. The results of these three studies indicated that CT053 cells were consistently tolerated in Chinese and North American patients with R/R MM, with significant and persistent clinical efficacy observed in patients with multiline therapy. The proliferation of cell was excellent in vivo with no anti-drug antibodies (ADA) detected.
CT053 has been approved as Orphan Drug by FDA in 2019 and Breakthrough Therapeutic Drug Designation by NMPA in 2020. CT053 will be submitted as a new drug to the NMPA in the first half of 2022 and as a BLA to the US FDA in the first half of 2023 for the treatment of MM treated with at least previous third-line therapies.
Claudin (CLDN) is one of the most important proteins for the tight connection of normal tissues. It has four transmembrane domains and is involved in physiological processes such as the regulation of paracellular permeability and electrical conductance. The CLDN18 family consists of at least 24 members, of which CLDN18.1 and CLDN18.2 are two isomers. Studies have shown that under normal physiological conditions, CLDN18.2 is only expressed on the surface of human gastric epithelial short-lived cells; However, it is highly expressed in gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer and other tumors. For example, the expression of this target exists in 50%-80% of gastric cancer patients. Therefore, CLDN18.2 may be an effective target for immunotherapy of solid tumors such as gastric cancer and pancreatic adenocarcinoma. Currently, there is no approved therapy for CLDN 18.2 worldwide.
CT041 is a humanized anti-CLDN 18.2 autologous CAR-T cell injection independently developed by CARSGEN Biotech, which is intended to be developed for the treatment of advanced gastric adenocarcinoma/esophageal and gastric junction adenocarcinoma, pancreatic cancer and other indications with positive CLDN18.2 expression and progression or recurrence after previous systematic treatment.
In the current phase I/II clinical trial in China and phase Ib clinical trial in the US, CT041 has shown good efficacy and safety in patients with CLDN18.2-positive gastric and pancreatic cancer. In one of the trials, CT041 showed a 50% ORR in 22 evaluable patients with gastric/gastroesophageal junction cancer, with a median progression-free survival (PFS) of 4.2 months and a median overall survival (OS) of 9.5 months.
CT041 was approved as an Orphan Drug designation by the US FDA in 2020 for the indication of Claudin 18.2-positive solid tumors.
AB011 is a humanized monoclonal antibody candidate that has been developed for the treatment of CLDN18.2-positive solid tumors. This is the second worldwide clinical trial approval for monoclonal antibody targeting CLDN18.2. Currently, a phase I clinical study of AB011 in the treatment of CLDN18.2-positive solid tumors is being conducted in China, and a phase II/III clinical trial for the main indication of gastric cancer/esophageal and gastric junction cancer is planned to be launched in the second half of 2022.
Glypican 3 (GPC3) is a carcinoembryonic antigen that is involved in cell proliferation, differentiation, migration, and apoptosis. It is rarely expressed in normal healthy tissues but is expressed in 70-80% of hepatocellular carcinomas. GPC3 is considered as a very promising target for tumor immunotherapy due to its tumor specificity.
CT011 is an autologous CAR-T candidate targeting GPC3 for the treatment of hepatocellular carcinoma. The product has been approved for a phase I trial in China, which is the first clinical trial of CAR-T cell therapy in solid tumors in China. Phase II trials are expected to begin in the second half of 2021.
CT017 is a next-generation autologous CAR-T candidate targeting GPC3 and contains transcription factors. Preclinical studies have shown that CT017 had the ability to remain and persist in non-lymphoid tissues such as solid tumors, thus showing enhanced anti-solid tumor efficacy. Currently, CT017 is in the stage of investigator-initiated trials to evaluate the safety and efficacy of GPC3-positive hepatocellular carcinoma in Chinese patients.
CD19 is a specific surface protein of normal and malignant B lymphocytes, which plays an important role in the development, proliferation, differentiation, and malignant transformation of B cells. Due to the specificity of CD19 expression in B lymphocytes and the universality of CD19 expression in malignant tumors, CD19 has become a potential molecular target for immunotherapy of B lymphocyte malignant tumors.
CT032 is an autologous CAR-T candidate for CD19 that combines a humanized CD19-specific single-strand fragment variant with the promise of a reduction in toxicity and immunogenicity. Currently, the product is undergoing an open-label, single-arm, phase I/II trial in China to evaluate the safety, efficacy, and cell kinetics of CT032 in the treatment of R/R B-cell non-Hodgkin's lymphoma (B-NHL).
CARSGEN Therapeutics has diversified its CAR-T layout, which includes both CLDN18.2, the first of its kind in the world, and CD19 and BCMA, which are very rare among CAR-T companies. Currently, most CAR-T companies are only conducting clinical trials in one or two hotspot targets.
As a conclusion, CARSGEN Therapeutics has the following advantages:
First, the world's first CLDN18.2 CAR-T candidate for solid tumors in the same category of drugs; Second, upgraded whole-human anti-BCMA CAR-T therapy has better safety; Third, it has the world's first CAR-T product candidate for the treatment of hepatocellular carcinoma with GPC3. Besides competitive products, CARSGEN Therapeutics also has its own platform and technology, which has higher flexibility and deeper research and development depth than the companies introduced through licensing.
CAR-T therapy represents a milestone in the field of cancer therapy. Since the launch of the first CAR-T therapy in 2017, five CAR-T therapies have been approved by the FDA, including Kymriah, Yescarta, Tecartus, and Breyanzi, which target CD19, and Abacma, which targets BCMA. Increasingly catching the intention of capital for CAR-T race tracking.
However, most approved CAR-T therapies target CD19, mostly in patients with hematologic malignancies. CARSGEN Therapeutics has not been focusing on the presence of products and competitors in the hematologic malignancies of the extensive layout. In addition to the CD19 targeting CT032 and BCMA targeting CT053 for hematologic malignancies, the rest of the product pipeline is all targeted at solid tumors. Its innovative and differentiated cell therapies have made CARSGEN Therapeutics a leader in the field of cell therapy in China.
CARSGEN Therapeutics has developed a differentiated pipeline of 11 product candidates and has received 7 IND licenses for CAR-T therapies in China, the United States and Canada, with the highest number of CAR-T therapies in China. Facing the unmet demand for cancer treatment of a huge amount of patients, CARSGEN Therapeutics focused on its original intention of challenging the difficulties in the CAR-T industry and develop more innovative drugs that are expected to cure cancer patients through continuous technological innovation.
1.The prospectus of CARSGEN Therapeutics
2.Results from Lummicar-2: A Phase 1b/2 Study of Fully Human B-Cell Maturation Antigen-Specific CAR T Cells (CT053) in Patients with Relapsed and/or Refractory Multiple Myeloma. Retrieved Nov 30,2020,from https://ash.confex.com/ash/2020/webprogram/Paper139802.html;
3.Retrieved Nov 19,2020, from http://www.carsgen.com/public/uploads/files/202011/72a9fb22a0cf221d.pdf;
4. Center for Drug Evaluation, NMPA
Retrieved Nov 30,2020, from
http://www.cde.org.cn/news.do?method=changePage&pageName=service&frameStr=21;
5.Katz B Z, Herishanu Y. Therapeutic targeting of CD19 in hematological malignancies: past, present, future and beyond[J]. Leukemia & Lymphoma, 2014, 55(5):999-1006.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


