PharmaSources/YuntianMay 31, 2021
Tag: Liver cancer , Immune Drug , Drug Combination
Liver cancer has recently drawn much attention by a Chinese movie star Ng Man-tat who died of liver cancer and the National Liver Day on Mar. 18. China is a big country in terms of liver cancer evidenced by the latest global cancer data 2020 released by the WHO: more than half of new and dead liver cancer patients globally are in China. As such, liver cancer drugs have been a highly valued pharmaceutical field in China, and immune drug combinations have also been receiving much attention as therapies that could bring more benefits to liver cancer patients.
Currently, two immune drug combination therapies for liver cancer have been approved in China, namely, atezolizumab + bevacizumab, and nivolumab + ipilimumab, separately for the first-line and second-line treatment. Furthermore, lenvatinib + pembrolizumab, and lenvatinib + nivolumab have both received clinical study approval for the first-line treatment. If you are looking for medical devices and supplies, then Pharmasources wound be your best choice.
| Combination regimen | Treatment | NMPA approval |
| Atezolizumab + bevacizumab | First-line treatment | Yes |
| Nivolumab + ipilimumab | Second-line treatment | Yes |
| Lenvatinib + pembrolizumab | First-line treatment | No |
| Lenvatinib + nivolumab | First-line treatment | No |
Fig. I Immune Drug Combination Therapies for Liver Cancer
The regimen of atezolizumab + bevacizumab is referred to as the T+A regimen and is the world’s first immune drug combination therapy approved for the first-line treatment of liver cancer.
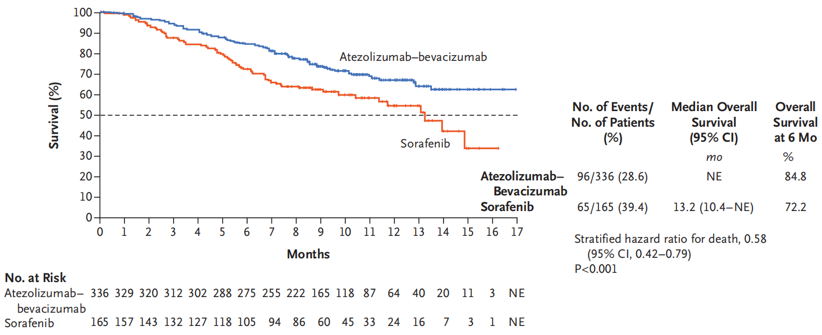
Fig. II T+A Regimen Significantly Prolonging the OS
IMbrave150 is an open-label, randomized, parallel-controlled, multicenter clinical study for the T+A regimen. The study compares the T+A regimen with sorafenib for the first-line treatment of patients with liver cancer. According to the interim analysis data firstly presented at the ESMO Asia Congress 2019, both the OS and PFS met predetermined statistical values; the median OS for the T+A regimen did not reach and that for sorafenib was 13.2 months, with 42% reduction in OS risks in the drug combination group, the median PFS for the T+A regimen was 6.8 months and that for sorafenib was 4.3 months, with 41% reduction in disease progression risks, and the ORR for the T+A regimen was 27.3% and that for sorafenib was 11.9%. Furthermore, the drug combination regimen also delayed the time of life quality deterioration as reported by patients.
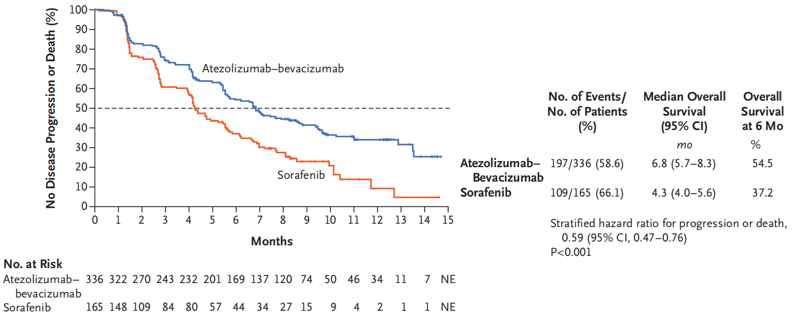
Fig. II T+A Regimen Significantly Prolonging the PFS
Based on the CheckMate-040 clinical study, the U.S. FDA was the first to approve the nivolumab + ipilimumab regimen on Mar. 11, 2020 for the second-line treatment of liver cancer patients previously treated with sorafenib.
Patients enrolled in the CheckMate-040 study are those with advanced HCC who are intolerant to or have progressed on sorafenib therapy, and BICR was assessed according to RECIST v1.1; for patients on the combination regimen, 8% achieved CR, 24% achieved PR, and DOR was 4.6 months to 30.5 months, of which 88% lasted at least 6 months, 56% lasted at least 12 months, and 31% lasted at least 24 months. And the safety of the combination regimen was good.
A. Lenvatinib + pembrolizumab regimen: This regimen, known as the “K+L combination”, has not been approved in China at present, however, it has been included in the latest China Anti-cancer Association Guidelines for Patients with Primary Liver Cancer. According to the KEYNOTE-524 lb study, the K+L combination was evaluated according to the mRECIST, with the ORR reaching 46.3%, mTTR reaching 2.4 months, mPFS reaching 9.7 months, and mOS reaching as high as 20.4 months.
B. Lenvatinib + nivolumab: According to the study of lenvatinib in combination with nivolumab for the first-line treatment of patients with unresectable liver cancer presented at the ASCO GI 2020, the ORR reached 76.7%, the DCR reached 96.7%, and the clinical benefit rate reached 83.3%, as evaluated according to the mRECIST, while according to the IRC assessment based on the RECIST 1.1, the ORR reached 54.2%, the DCR reached 91.7%, and the CBR reached 62.5%.
As a “punch combination” for liver cancer treatment, combination regimens have shown great advantages in the treatment, and combination regimen has become a new treatment option for patients especially when the effects of monotherapies are not good. Furthermore, as more and more immune drugs are approved for liver cancer, more immune drug combination regimens will emerge in the future, which will greatly improve the current severe situation of liver cancer treatment.
1. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma,2020;
2. Guidelines of Chinese Society of Clinical Oncology (CSCO) Hepatocellular Carcinoma (2020);
3. China Anti-cancer Association Guidelines for Patients with Primary Liver Cancer
Yuntian, Ph.D. in medicinal chemistry, is mainly engaged in small molecule drug research, especially good at small molecule drug synthesis process and later stage drug development research. He has completed the synthesis and activity evaluation of multiple anti-cancer drug molecules.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


