Deepak HegdeMarch 11, 2021
Tag: Continuous Manufacturing , Generics , Big Pharma , API
Continuous Manufacturing, an innovation in the manufacturing field has been gaining importance over the last few years, as drug products have been getting approved by regulators over the world and reaching the market. This series of three article on this topic reviews this important topic.
The first article provides an outline of the subject and highlights the advantages of continuous manufacturing and shows how innovative drug companies and generic drug companies are approaching continuous manufacturing.
The second article focuses on the manufacturing of Active Pharmaceutical Ingredients (API’s) and drug products using the continuous manufacturing technique and some of the challenges associated with continuous manufacturing.
The third article shows the definition of batch in continuous manufacturing, its importance from a regulatory perspective, the importance of linking it to control strategies and why continuous manufacturing presents a major shift for quality systems. It also reviews the global regulatory landscape associated with continuous manufacturing.
ABSTRACT: This paper presents an outline to the topic of continuous manufacturing. It outlines the applicability of technique from development to commercial manufacturing and the potential benefits of adopting continuous processing technologies for by companies involved in the manufacture of both, innovative and generic pharmaceutical products. Finally, it highlights some of the issues faced by generic companies while adopting continuous manufacturing technique.
As a concept, continuous manufacturing has been around for certain industries like oil and gas for a very long time and manufacturing is run in a continuous mode. However, in case of the pharmaceutical industry, continuous manufacturing has emerged as an innovation in the manufacturing space in the past decade and a half. This innovation has the potential to improve flexibility, speed, and robustness in the manufacture of pharmaceuticals. As is the case with deployment of any new technology, there have been apprehensions from a technological, commercial, and regulatory point of view. However, with the passage of time and products being approved on the global market, this technology has been gaining acceptance globally.
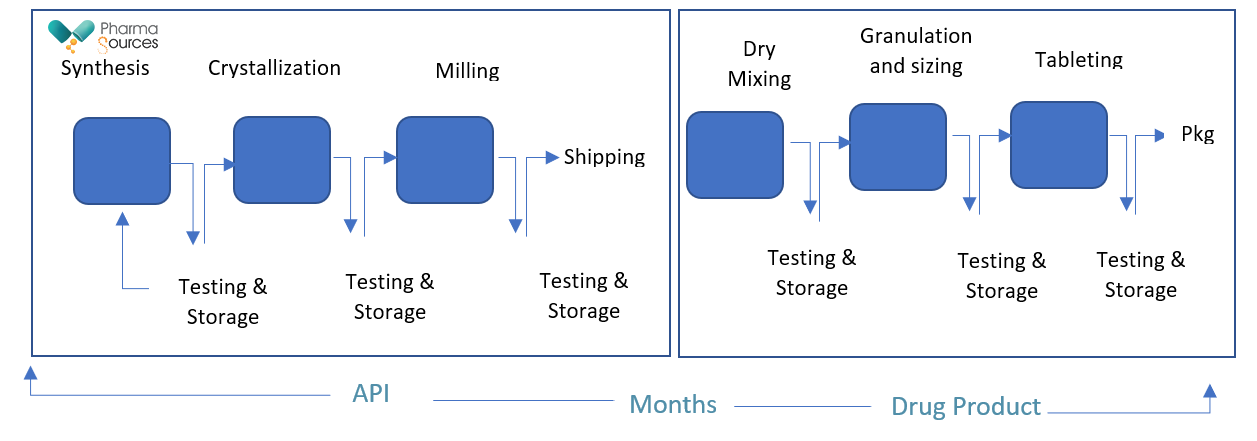
Traditionally, the manufacture of pharmaceuticals followed a batch process, both in case of API and drug product manufacturing. Fig. 1 shows the representative unit operations in batch process of manufacturing
Fig. 1: Batch Manufacturing process
(Schematic adapted from “Modernizing Pharmaceutical Manufacturing:
from Batch to Continuous Production S. Lee et al., J. Pharm. Innov., 2015”)
Based on the number of unit operations and capabilities available at site of manufacturing, the entire process of manufacturing from API to drug product can take a few days to a few months1. In this, the product is collected after every unit operation, tested offline after every operation, because of which actual processing time can vary from a few weeks to few months.
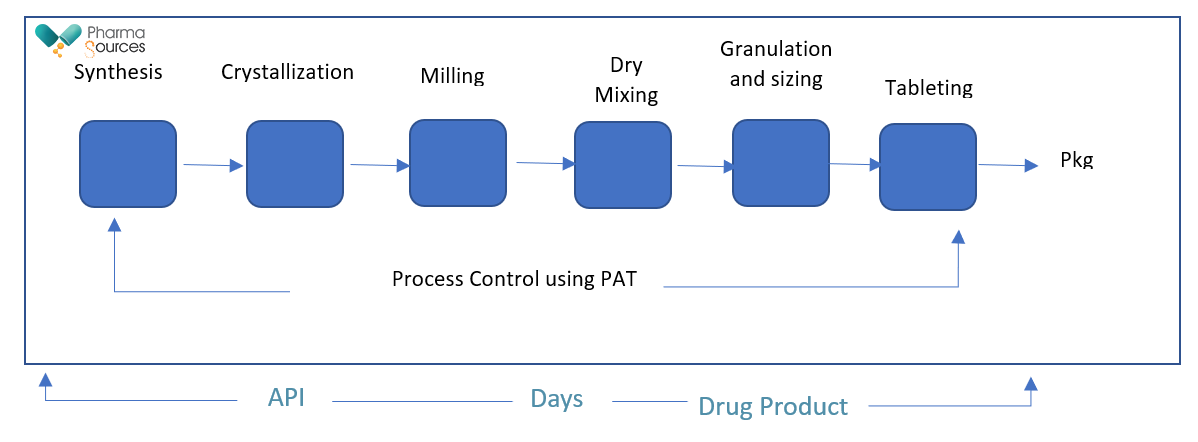
Comparatively, the manufacture of pharmaceuticals following a continuous manufacturing process, both in case of API and drug product manufacturing is shown below in Fig. 2 reflecting the unit operations.
Fig. 2: Continuous Manufacturing process
(Schematic adapted from “Modernizing Pharmaceutical Manufacturing:
from Batch to Continuous Production S. Lee et al., J. Pharm. Innov., 2015”)
At one site 1) Using smaller equipment 2) Shorter supply chain
In a continuous process, the material(s) and product are continuously charged into and discharged from the system, respectively, throughout the duration of the process. Using smaller equipment, entire process of manufacturing from API to drug product can be completed in a few days using continuing manufacturing2.
As shown in Fig. 3, continuous manufacturing is a concept that can be applied across the lifecycle of development of a product from product development to commercial manufacturing. Bio pharmaceuticals companies have started applying continuous manufacturing from a stage of early development of products and adapted their research and development laboratories to fully apply this concept.
Fig 3: Applicability of Continuous Manufacturing process

Application of continuous manufacturing at a development stage
• Enables full utilization of Quality by Design (QbD).
• Allows efficient utilization of resources to support process development, process development process scale-up and technology transfer.
• Allows overall reduction in API consumption compared to batch equipment.
• Simplifies and robust process transfer and scale-up.
• Increases process understanding.
Application of continuous manufacturing at a commercial stage
• Allows a smaller equipment footprint which in turn allows more flexibility, lower costs, and is environmentally friendly.
• Allows an integrated process with fewer separate unit operations.
• Enables real time product quality information leading to real-time-release-testing (RTRT)
• Allows better process control and robustness leading to lower batch-to-batch variation.
• Allows greater flexibility of batch size.
• Makes it easier to scale up/down to accommodate changing supply chain needs.
Continuous manufacturing is being adopted by both Big Pharmaceutical companies focusing on development of innovative products as well as companies engaged in development of generic products. However, the reasons for both these types of companies adopting continuous manufacturing slightly different.
Big Pharma companies are adopting continuous manufacturing as
• It allows faster production.
• Leads to more reliable products.
• It allows better flexibility in manufacturing volumes.
• Allows reduced carbon footprint.
Generic companies are adopting continuous manufacturing as
• Allows them to focus on high volume products.
• Allows them improvement of yields.
• Allows lower costs of manufacturing.
• Increases production capacity.
Generic manufacturers constantly need to take measures to keep Cost of Goods Sold (COGS) low and effective regulatory change management is a major requirement for any generic company. A major challenge is that during the development phase of products, a lot more development work is needed. Generic products are often developed for multiple geographical markets, and the regulatory requirements for submission vary across the geographies3. This makes it difficult for generic companies to make any significant changes to approved procedures as any delay in the approval process would have adverse impacts on their market presence and share in the highly competitive environment. The Association for Accessible Medicines (AAM), which represents generic drug manufacturers has raised concerns about the costs associated with continuous manufacturing4. AAM estimated equipment and facility costs at $50 million per 1 billion units produced when employing continuous manufacturing. $30 million for facilities and $20 million for equipment (facilities reconfiguration, HVAC and other mechanical/electrical retrofit).
Some potential drawbacks and issues associated with continuous manufacturing for generic companies which have been highlighted are:
• Long technology integration times to switch from a batch to a Continuous Manufacturing process.
• A significant portion of batch equipment is still within useful life.
• The risk of single dedicated/integrated lines for key products.
• Significant set-up cleaning changeover time for small batch products requiring multiple lines of large and small capacity with modifications needed between products.
• Significant up-front resources, both human and financial, to establish integrated in-line testing systems.
• More and more recent generic molecules fall into the low volume category
• Shift in product portfolios of companies from small molecules to focus on increasing numbers of biologics and gene therapies which threaten the need for larger capacity facilities.
• The generic industry currently is not confident about the acceptance of non-traditional methods in non-U.S. markets.
1. FDA Perspective on Continuous Manufacturing, Sharmista Chatterjee, IFPAC Annual Meeting, Baltimore, Jan 2012
2. Modernizing Pharmaceutical Manufacturing: from Batch to Continuous Production S. Lee et al., J. Pharm. Innov., 2015
3. Is Continuous Manufacturing A Good Fit for Generic Drug Products? Ajay Pazhayattil, Marzena Ingram, Ramana Kumar, Sudhakara Rao, and Robin Meier, Pharmaceutical Online, Guest Column , July 25, 2019
4. Pharma Industry Weighs in on Continuous Manufacturing, Patricia Van Arnum, DCAT Value Chain Insights, June 2019

Deepak Hegde, Ph.D., M.F.M, is an industrial pharmacist by training. He has a been involved in development and commercialization of both innovative and generic drugs from a very early phase of development to technical transfers for commercial manufacturing sites, for the past 25 years. During his career, he has worked Rhone Poulenc, Novartis (Sandoz), USV Ltd., WuXi AppTec and GSK. He is currently working with EOC Pharma. as Chief Technology officer.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


