PharmaSources/zhulikou431February 08, 2021
Tag: GMP , Inspection , Trend
International GMP compliance inspections in the pharmaceutical industry decreased significantly in 2020 as affected by the COVID-19 pandemic. Due to the transportation restrictions and pandemic prevention policies of countries, the frequency of international inspections decreased. Regulatory authorities are using more written designs or remote assessments. Based on the inspection data of China, the U.S., and the EU in 2020, below is a review of the inspection trends in 2021 in the hope of providing a reference for pharmaceutical practitioners.
Part I: Summary and Analysis of FDA Inspection Data
The FDA’s inspections on foreign enterprises significantly decreased in 2020 due to the impact of the pandemic. According to the data released so far, the FDA mainly focused on the compliance inspections on the U.S. pharmaceutical manufacturers. The latest data publicly available on FDA's official website shows that it issued six warning letters to Chinese enterprises in 2020, which was a lot fewer than the 15 warning letters in 2019, 19 warning letters in 2018, and 22 warning letters in 2017.
Table 1: Warning Letters Issued by the FDA to Chinese Enterprises in 2020
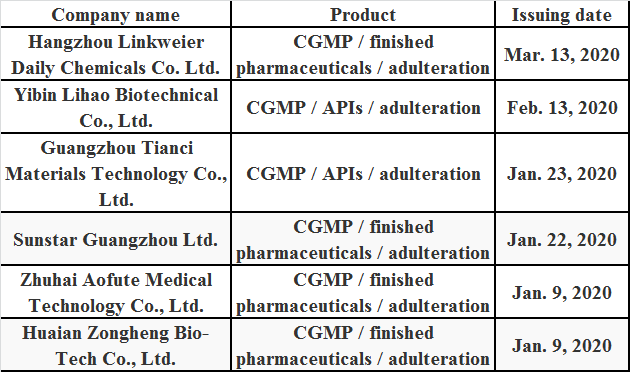
As seen from the warning letters, the GMP standard of some daily chemical and medical product enterprises needs to be improved, for example, deficiencies in the progress of quality management of the products. These deficiencies were common in warning letters to many companies, such as failure to conduct proper laboratory detections for each batch of drug products to determine whether they meet the final drug quality standards, including active ingredient and content identity, failure of detecting labeled active ingredients before releasing the drug products, and failure in the active ingredient identification and content determination of some products.
In 2020, the FDA issued more warning letters to Indian enterprises than to Chinese enterprises. However, further analysis shows that most of the site inspections involved in these warning letters occurred in 2019, namely, before the outbreak of the COVID-19 pandemic.
Part II: Data of EU Non-Compliance Reports (NCRs)
According to the data published in the EU GMP database, the EU issued four NCRs in 2020, involving countries including Switzerland, Austria, Poland, and India.
Table 2: EU NCRs in 2020
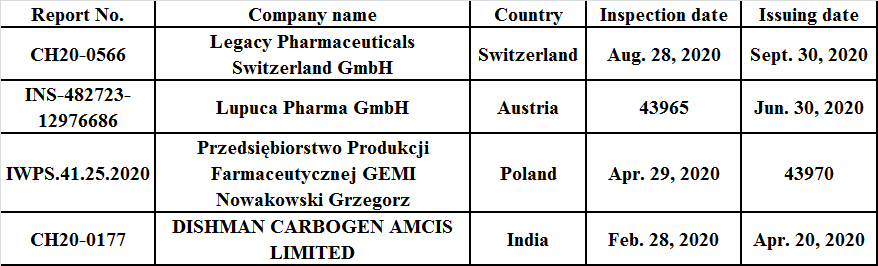
Through analysis of the above NCRs, the main deficiencies include:
Insufficient control over the air quality of clean rooms, incomplete verification of the air handling system, insufficient validation of sterile filtration operations, inadequate frequency of media fills (less than 2x/year) on some of the production lines, deviations occurred during the media fills were not coped with promptly, inadequate deviation management, etc., while the company’s quality systems were functional, there was a lack of management supervision. Therefore, the company’s sterile manufacturing management is considered not in compliance with GMP overall.
The company's manufacturing qualification license is limited. Based on the CAPA evaluation of the company, the manufacturing qualification license cannot be prolonged. Major systemic deficiencies that could not be resolved were in the areas of supplier qualification, change management, deviation management, qualification of equipment, validation of computerized systems, and good documentation practice.
Failure to take steps to prevent microbial and mold contamination and cross-contamination, use measures to properly investigate contamination during the progress of monitoring and quality control, equipment and plant were not properly cleaned and maintained. The manufacturer did not have adequate storage areas to ensure that the drugs were under control in the production processes.
The company’s approach to material management, including the label traceability, storage conditions, dispensing and cleaning, pest control of raw materials, intermediates, solvents, and recovered solvents, was considered not in compliance with EU GMP. The company failed to lower the risks of cross-contamination in multipurpose facilities and was not aware of the necessary measures to be taken before introducing new chemical entities in the areas of sampling, distribution, and synthesis. The recycling of solvents was not properly managed and documented. Shortcomings were observed concerning cleaning validation and process validation. The critical and major deviations were not effectively resolved.
With the continuous development of the COVID-19 pandemic, the EU’s cross-border inspections were also affected. As a result, the EU released guidance regarding distant assessments to encourage member states to actively take new assessment measures to ensure that pharmaceutical manufacturers and bio pharmaceuticals companies comply with EU GMP, for example, Germany used distant assessments for Zhejiang Jingxin and eventually confirmed that Zhejiang Jingxin complied with EU GMP.
Part III: Summary of NMPA Inspection Data
The NMPA has an annual pharmaceutical product inspection plan outside of China every year. With the outbreak of the COVID-19, the CFDI under the NMPA did not release an inspection plan outside of China in 2020, and it might have made a plan on the new inspections based on the ones left from the previous year and combined risk assessment.
According to the NMPA announcement, the NMPA suspended the import of methoxyphenamine hydrochloride from Sanyo Chemical Laboratories Co., Ltd., and the drug administration at each port suspended the issuance of import customs clearance forms for the product in Aug. 2020. It was mainly because Sanyo Chemical Laboratories Co., Ltd. turned down the NMPA’s onsite inspection of its methoxyphenamine hydrochloride production site. As a result, the enterprise was deemed as refusing the inspection according to the Provisions on the Administration of Overseas Inspection of Drugs and Medical Devices, and its production process was directly determined as not complying with the requirements of China’s Good Manufacturing Practice for Drugs (2010 Revision).
Furthermore, the NMPA suspended the import, sale, and use of Nifuratel Tablets and Nifuratel Vaginal Tablets of POLICHEM S.R.L. in Sept. 2020. It was mainly because the overseas inspection conducted by the NMPA on the company found that the company failed to, as required, submit a rectification plan and carry out effective rectification on the deficiencies found of nifuratel products, and the actual technical process of the Nifuratel Vaginal Tablets product was not fully consistent with the declared process, which affected the key quality attributes of the product and did not meet the requirements in Good Manufacturing Practice for Drugs (2010 Revision) of China.
Meanwhile, many onsite inspections of CFDI in China were also affected due to restrictions of business trips within the country during the early stage of the COVID-19 outbreak. The CFDI, to close these gaps and keep the work, has actively cooperated with the inspection authorities of provincial administrations, to complete the inspection tasks of 2020 in various ways.
The CFDI released the Progress of the CFDI’s Joint Inspection of Drug Registration in 2020 (by the end of November) on Dec. 7, 2020, which shows the updated data of inspections in China:
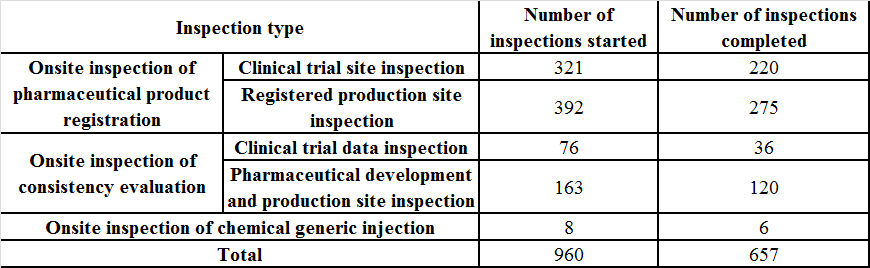
Part IV: Future Trend Preview
Through the summary and analysis of the data above, it is predicted that the inspections in and outside China will show the following characteristics in 2021:
Firstly, more agencies will complete compliance inspections through written designs or distant assessments, such as the FDA, the EU, and the WHO;
Secondly, the corresponding certificate validity periods of enterprises that pass written or distant assessments will be shortened, for example, the validity period is tentatively set as one year;
Thirdly, the CFDI’s inspections on enterprises in China will remain regular;
Fourthly, the CFDI’s overseas inspections will be based on written assessments in
the first half of 2021 and gradually use onsite inspections in the second half of
2021.
References
1- NMPA official website data
2- CFDI official website data
3- EMA official website data
4- FDA official website data
5- WHO official website data
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


