Neeta RatanghayraOctober 22, 2020
Tag: Artificial Intelligence , pharmaceutical industry , china
Artificial intelligence (AI) refers to the use of automated algorithms to perform the tasks conventionally done with human intelligence. Over the last few years, the use of artificial intelligence in the pharma and biotech industry has redefined how scientists develop new drugs and manage diseases.
AI is set to impact China’s pharmaceutical health supplies and biopharma industry significantly. Under China’s strategic plan, ‘Made in China 2025’, the Chinese government aims to heavily invest in research and development activities to boost innovation. One of the key areas of focus is using machine learning algorithms to reduce research times and costs, which would help companies have a robust and sustainable drug pipeline and enable increased trial completion rates. Therefore, AI is becoming more and more important in making equipment for pharmaceutical industry for many medical supplies manufacturers and medical device sales companies.

Artificial intelligence in new drug development
Traditional methods to develop new drugs take an average of 10-14 years and a large amount of money. When AI is introduced into the research and development phase, machines take the place of researchers to design the drugs - this increases efficiency and results in a considerable reduction in time and costs.
AI algorithms allow researchers to quickly identify the best drug candidate for a target or a molecular compound from a pool of drug candidates. AI models can also simulate and predict the experimental and clinical performance of the newly developed drug.
AI can also predict the possible side effects of a drug before clinical tests, which can help avoid potential issues beforehand. In case problems arise, AI can be used to modify the molecular structure of the drug.
Tencent, a multinational tech company in China, has launched an AI-driven drug discovery platform called iDrug, which helps drug developers significantly reduce the time and cost involved in preclinical drug discovery. iDrug is a platform based on deep learning algorithms. iDrug provides database and cloud computing, covering phases of the preclinical new drug development, including protein structure prediction, virtual screening, molecular design/ optimization, ADMET property prediction, and synthetic route planning.
Hong Kong, S.A.R., China-based Insilico Medicine is working to develop a high-performance AI platform for drug development. Insilico Medicine, in collaboration with the Chinese CRO Wuxi and the University of Toronto, conducted a study to identify lead candidates using AI. The study, published in Nature Biotechnology in 2019, proved that AI technology could help identify compounds targeting discoidin domain receptor 1 (DDR1), a protein expressed in epithelial cells, and associated with fibrosis. The AI system helped identify six substances that blocked the protein, and, of this, one was found to be effective in treating mice with renal fibrosis. The whole process of identification was completed in three weeks – much faster than the traditional drug discovery process, which involves testing thousands of substances over a decade and can cost from US$500 million to US$2.6 billion.
AI in drug repurposing
Drug repurposing is a process through which existing drugs are used to treat emerging and challenging diseases. Drug repurposing is a promising approach because of the opportunity for reduced development timelines and overall costs.
Hyper-dimension, a Beijing-based pharma company, is using AI to find a possible cure for COVID-19. It is currently working with the Institute of Materia Medica under the Chinese Academy of Medical Sciences to use AI to find a treatment for COVID-19 among existing drugs. Hyper-dimension is using AI to run the testing model and see what kind of drug matches the protein of the novel coronavirus. Once the drug is fixed, they conduct further assays to test the drug efficacy.
AI in clinical trials
As per a Cognizant report published in 2015, around 80% of clinical trials fail to meet enrollment timelines, and nearly one-third of Phase III clinical study terminations are due to enrollment difficulties. AI can help in extracting and making sense of clinical data. IBM Watson™ for Clinical Trial Matching enables clinicians to find a list of clinical trials for an eligible patient quicker and easier than the conventional methods.
Companies are also using natural language processing to screen patients for drug trial enrolment. Harbour BioMed, a leading biopharmaceutical company in China, successfully completed the Phase II clinical trial of its innovative biological drug, tanfanercept. The trial tested the safety and efficacy of tanfanercept in patients with moderate to severe dry eye. A striking feature of this trial was that the trial duration was significantly shortened with the help of AI. In the study, Harbour BioMed and Happy Life Tech, a Chinese medical AI company, worked with a group of ophthalmologists to build behavior profiles for disease diagnosis and treatment. Using disease models processed by AI-assisted platforms, they performed multi-dimensional patient flow analysis, leading to the accurate location of the potential patient groups and target hospitals. This, along with analysis of patient compliance, allowed precise recruitment and enrolment plans, which helped shorten the trial duration.
Artificial intelligence-based drug discovery companies in China
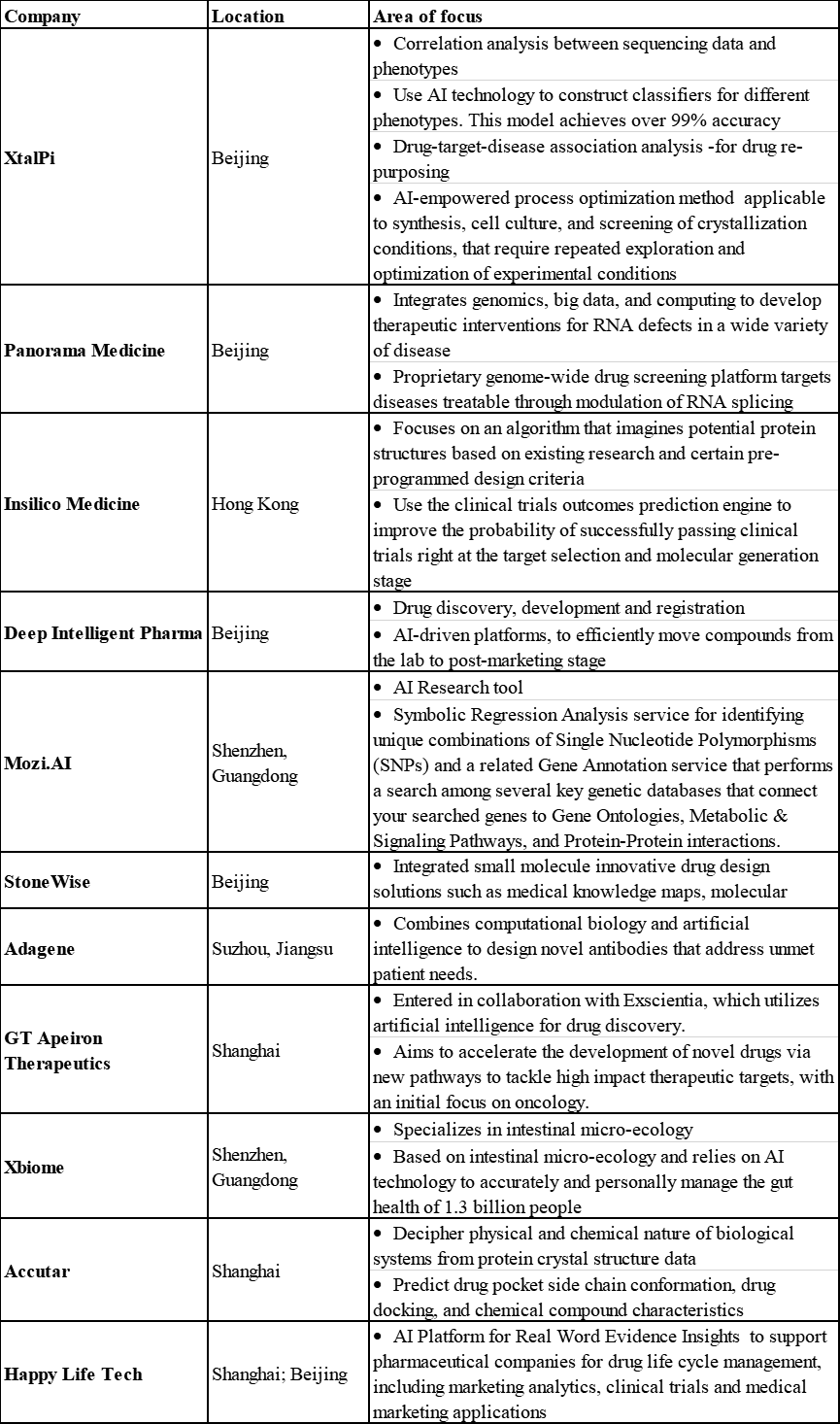
In China, several companies and start-ups are working to explore AI technology in drug discovery. Below is a list of selected companies in China.
Challenges to the adoption of artificial intelligence in the pharma industry
Though the benefits of artificial intelligence are well known, very low proportion of companies use or invest in AI technologies in facilitating drug discovery and development. Some of the key challenges which the companies face are:
· IT infrastructure based on legacy systems, not designed with AI in mind.
· The systems lack the capacity to store large databases; they also interoperability.
· The majority of data within medical systems is in free form which cannot be processed and used efficiently by health professionals.
· Hesitancy to use new technology - Though AI technologies such as machine learning and smart automations have been available for a while, they are still viewed as a relatively new technology.
China has identified AI as a strategic national industry
Artificial intelligence helps reduce costs, create new, effective treatments, which would ultimately save lives. AI is being leveraged to streamline China’s overburdened health care system which is facing imminent crisis due to its aging population, lack of qualified doctors and regional imbalance in healthcare facilities.
Even though the use of AI in the pharma and biotech industry is still in its infancy, exploring innovative drugs through AI represents the future trend of the industry, and several AI companies in China are springing up and have started exploring the sector.
References
1. Zhou Y, Wang F, Tang J, Nussinov R, Cheng F. Artificial intelligence in COVID-19 drug repurposing [published online ahead of print, 2020 Sep 18]. Lancet Digit Health. 2020;doi:10.1016/S2589-7500(20)30192-8
2. AI lends speed and precision in the urgent search for new drugs. 2020. Available at: https://www.nature.com/articles/d42473-020-00093-0. Accessed on: 14October2020.
3. Patients Recruitment Forecast in Clinical TrialsAccurate. 2015. Available at: https://www.cognizant.com/whitepapers/patients-recruitment-forecast-in-clinical-trials-codex1382.pdf. Accessed on: 16 October 2020.
4. AI key to medical innovation. China daily. 2020. Available at: https://global.chinadaily.com.cn/a/202008/13/WS5f348d4ca31083481725ff65.html Accessed on: 16 October 2020.
5. XtalPi. Available at: https://www.xtalpi.com/en/. Accessed on: 16 October 2020.
6. Panorama Medicine. Available at: https://www.panoramamedicine.com/. Accessed on: 16 October 2020.
7. Deep Intelligent Pharma. Available at: https://deepintepharma.com/. Accessed on: 16 October 2020.
8. Insilico medicine. Available at: https://insilico.com/. Accessed on: 16 October 2020.
9. Mozi.AI. Available at: https://mozi.ai/. Accessed on: 16 October 2020.
10. Stonewise. Available at: http://www.stonewise.cn/home. Accessed on: 16 October 2020.
11. Adagene. Available at: https://www.adagene.com/. Accessed on: 16 October 2020.
12. GT Apeiron Therapeutics. Available at: http://www.apeiron-bio.com/about-gt-apeiron-therapeutics/. Accessed on: 16 October 2020.
13. Xbiome. Available at: https://www.xbiome.cn/. Accessed on: 16 October 2020.
14. Accutar Biotech. Available at: https://www.accutarbio.com/. Accessed on: 16 October 2020.
15. Happy Life Tech. Available at: https://www.happylifetech.com/. Accessed on: 16 October 2020.
About the Author:
Neeta Ratanghayra is a freelance medical writer, who creates quality medical content for Pharma and healthcare industries. A Master’s degree in Pharmacy and a strong passion for writing made her venture into the world of medical writing. She believes that effective content forms the media through which innovations and developments in pharma/healthcare can be communicated to the world."


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


