Neeta RatanghayraNovember 05, 2020
Tag: mRNA Vaccine , COVID-19 Vaccine , mRNA Delivery Technologies
In early January this year, Chinese authorities shared the genetic sequence of SARS-CoV-2, the novel coronavirus.1 With this information, researchers identified the sequence for the spike protein, a key protein found on the surface of the virus. The spike protein showed the potential to be a viable vaccine candidate and formed the basis for messenger RNA (mRNA) vaccine development for COVID-19.
mRNA vaccines platforms are one of the most promising technologies harnessed to develop a COVID-19 vaccine. Moderna, which specializes in mRNA therapeutics, became the first company to test a vaccine against the novel coronavirus in humans. Moderna’s mRNA vaccine candidate has entered phase two of clinical trials, representing an important milestone in bringing the vaccine closer to the markets.
How do mRNA Vaccines Work?
mRNA vaccine platforms work on a fascinating concept - in these platforms, an in vitro transcribed (IVT) mRNA sequence that encodes a disease-specific antigen is introduced into the cell. Once inside the cell, the mRNA is translated into the target protein, in this case, the antigen, to trigger the body’s immune system. The IVT mRNA is engineered to resemble a fully processed mature mRNA molecule as they occur naturally. Thanks to advances in technology, the mRNA can be engineered to be more stable and highly translatable through various modifications. As the final step, IVT mRNA is degraded by normal physiological processes, reducing the risk of metabolite toxicity.2
The History of mRNA Vaccines - Initial Findings Were Met With Skepticism
The effectiveness of mRNA vaccines for direct gene transfer was first reported by Woff et al. in 1990. Woff and colleagues demonstrated that “naked DNA,” that is, plasmid DNA formulated without transfecting agents, could be directly injected into mouse muscle with resultant expression of the encoded protein by cells. The researchers demonstrated that naked RNA could similarly result in the in vivo expression of encoded protein; however, more attention focused on utilizing plasmid DNA, rather than mRNA, likely because of concerns about the instability of mRNA.3
In 1992, a study by Bloom et al. demonstrated that the administration of vasopressin-encoding mRNA in the hypothalamus could elicit a physiological response in rats. In 1993, liposome-formulated mRNA was shown to generate influenza-specific cytolytic T cells in mice. However, due to the potential concerns with mRNA instability, high innate immunogenicity, and inefficient in vivo delivery, these early promising results did not lead to substantial investment in mRNA therapeutics, and more emphasis was laid on DNA-based and protein-based therapeutics.4,5
Two Major Developments Changed the Perception towards mRNA
Though many mRNA studies showed promising results, much emphasis was on DNA based technologies, which explained the difference in excitement about the technologies. However, two developments changed the perception and reality of mRNA. In 2005, Weissman and Kariko found that modified nucleosides made IVT mRNA less immunogenic. In a follow-up study, they showed that using pseudouridine instead of uridine resulted in mRNA that was more stable and had increased translational capacity. The use of modified nucleosides thus addressed critical issues for mRNA—stability of the mRNA, increased production of the encoded protein, and some decrease of the innate immunogenicity.6,7
What Makes mRNA Vaccines an Attractive Option for COVID-19?
The ability to make an mRNA construct by merely knowing the genetic sequence of the desired antigen makes mRNA a relatively faster technology to produce a vaccine for a pandemic like COVID-19.
And if you are looking for mRNA Vaccines or other medical devices and supplies, then PharmaSources could help you. Apart from medical devices and supplies, PharmaSources also provides pharmaceutical water systems for customers. If you are interested in pharmaceutical packaging machinery, you can also contact with PharmaSources.
The following characteristics make mRNA an excellent option for vaccine development:8
· Rapidity of making constructs - mRNA can be designed for efficient delivery using carrier molecules, allowing rapid uptake and expression in the cytoplasm.
· Non-infectious, non-integrating platform - mRNA is a non-infectious, non-integrating platform; hence, there is no risk of infection or insertional mutagenesis.
· Relatively temporary presence in vivo - The in vivo half-life of mRNA can be modulated by various modifications and delivery routes. Once inside the cell, mRNA is degraded by normal cellular processes.
· Safety - The inherent immunogenicity of the mRNA can be down-modulated, which enhances its safety profile.
· Generic and rapid manufacturing processes compared to drugs or recombinant proteins - mRNA can be produced rapidly due to the high yields of in vitro transcription reactions, enabling flexibility to scale up production. This feature is pivotal to meet the demands of a pandemic.
mRNA Delivery Technologies
Conquering the cytoplasmic membrane barrier and avoiding digestion by RNases are pivotal for RNA delivery into target cells. Lack of toxicity and immune stimulation are other vital aspects for optimal delivery.9
Apart from the physical delivery modes such as gene guns and electroporation, mRNA vaccines can be delivered into the cytoplasm by cationic lipids and polymers. Novel delivery platforms, especially lipid nanoparticles (LNPs), are increasingly utilized to avoid the limitation of toxic chemical transfection reagents.
Lipid nanoparticles facilitate efficient delivery of RNA and dramatically enhance antigen expression; hence, they are among the most commonly used delivery vehicles. LNPs mostly consist of four components:10
· An ionizable cationic lipid, which enables self-assembly into virus-sized particles and facilitates the release of mRNA to the cytoplasm
· Lipid-linked polyethylene glycol which increases the half-life of formulations
· Cholesterol, which acts as a stabilizing agent
· Naturally occurring phospholipids to support lipid bilayer structure
By varying the route of administration, the magnitude and duration of protein production from mRNA–LNP vaccines can be controlled. Compared to systemic routes, intramuscular and intradermal routes have been reported to result in more persistent protein expression.
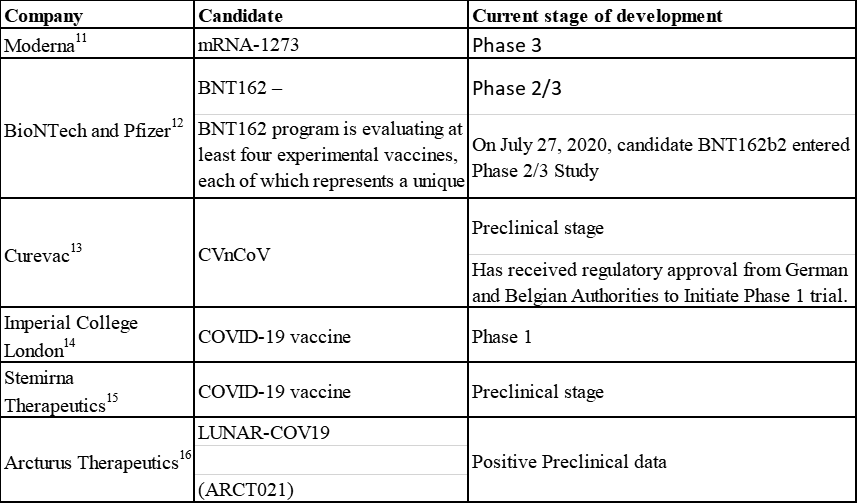
Companies Exploring mRNA Vaccines for COVID-19
Moderna’s mRNA vaccine candidate, mRNA-1273, demonstrated positive interim Phase 1 data in May this year and on July 27, 2020 Moderna's candidate entered phase 3 trial. BioNTech and Pfizer vaccine candidate, BNT162b2, also entered phase 2/3 on the same day as Moderna.11,12
Apart from Moderna, many other companies are testing mRNA vaccines, and their candidates are at different phases of clinical development. Below is a list of selected companies exploring mRNA vaccines for COVID-19:
mRNA Technology – Potential to Transform Medicine
Numerous preclinical and clinical studies have demonstrated the efficacy of mRNA vaccine platforms. While animal studies have generated great optimism, results from clinical trials of mRNA vaccines are somewhat modest, with efficacy highly dependent on the dose and route of administration. Nevertheless, these trials represent only some variations of mRNA vaccine platforms, and there may be considerable differences when the expression and immunostimulatory profiles of the vaccine are modified.9
The innovation behind mRNA platforms will enable researchers to develop novel vaccines rapidly; however, exploring strategies to optimize vaccine design and enable efficient development is key to success and can make or break the plan. For example, the improper incorporation of modified nucleosides can negatively impact the transcription products, which, in turn, can increase costs.9
mRNA technologies have the potential to transform medicine - Despite the challenges with vaccine development, companies are showing great enthusiasm, and enormous investments have been made to come out with a viable solution for this challenging phase.
References
1. Novel Coronavirus – China. Disease outbreak news : Update. WHO. 12 January 2020. Available at: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed on: 30 July 2020.
2. Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759-780. doi:10.1038/nrd4278
3. Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465-1468. doi:10.1126/science.1690918
4. Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255(5047):996-998. doi:10.1126/science.1546298
5. Martinon F, Krishnan S, Lenzen G, et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23(7):1719-1722. doi:10.1002/eji.1830230749
6. Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165-175. doi:10.1016/j.immuni.2005.06.008
7. Karikó K, Muramatsu H, Welsh FA, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833-1840. doi:10.1038/mt.2008.200
8. Liu MA. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines (Basel). 2019;7(2):37. Published 2019 Apr 24. doi:10.3390/vaccines7020037
9. Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA Vaccines for Infectious Diseases. Front Immunol. 2019;10:594. Published 2019 Mar 27. doi:10.3389/fimmu.2019.00594
10. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261-279. doi:10.1038/nrd.2017.243
11. Phase 3 clinical trial of investigational vaccine for COVID-19 begins. News Releases. July 2020. Available at: https://www.nih.gov/news-events/news-releases/phase-3-clinical-trial-investigational-vaccine-covid-19-begins. Accessed on: 30 July 2020.
12. Pfizer and BioNTech Choose Lead mRNA Vaccine Candidate Against COVID-19 and Commence Pivotal Phase 2/3 Global Study. Pfizer. News. July 2020. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-choose-lead-mrna-vaccine-candidate-0. Accessed on: 30 July 2020.
13. CureVac Receives Regulatory Approval from German and Belgian Authorities to Initiate Phase 1 Clinical Trial of its SARS-CoV-2 Vaccine Candidate. Curevac. Press Release. June 2020. Available at: https://www.curevac.com/news/curevac-receives-regulatory-approval-from-german-and-belgian-authorities-to-initiate-phase-1-clinical-trial-of-its-sars-cov-2-vaccine-candidate. Accessed on: 30 July 2020.
14. Imperial COVID-19 vaccine trial expands to additional sites. Imperial College London. News. July 2020. Available at: https://www.imperial.ac.uk/news/200435/imperial-covid-19-vaccine-trial-expands-additional/. Accessed on: 30 July 2020.
15. Stemirna. Pipeline. Available at: http://www.stemirna.com/en/rd/index.aspx. Accessed on: 30 July 2020.
16. Arcturus Reports Positive Preclinical Data for its COVID-19 Vaccine Candidate. Arcturus Therapeutics. News Releases. April 2020. Available at: https://ir.arcturusrx.com/news-releases/news-release-details/arcturus-reports-positive-preclinical-data-its-covid-19-vaccine. Accessed on: 30 July 2020.
About the Author:
Neeta Ratanghayra is a freelance medical writer, who creates quality medical content for Pharma and healthcare industries. A Master’s degree in Pharmacy and a strong passion for writing made her venture into the world of medical writing. She believes that effective content forms the media through which innovations and developments in pharma/healthcare can be communicated to the world."
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


