(July.27th-31th)
18. Clinical trials were approved for recombinant human papillomavirus 9-valent vaccine (Pichia pastoris) and recombinant herpes zoster vaccine (CHO) jointly applied for by Shanghai Easyway and Ab&b Bio-Tech on July 27, with the latter being the first Chinese recombinant herpes zoster vaccine approved for a clinical trial.
19. Shanghai Bovax Biotechnology initiated a phase III clinical trial of recombinant human papillomavirus 9-valent (types 6, 11, 16, 18, 31, 33, 45, 52, 58) vaccine (Hansenula polymorpha) on July 27 to evaluate its immunogenicity and safety in Chinese females aged 16 to 26.
20. AbbVie announced on July 29 that its JAK inhibitor: Rinvoq plus topical corticosteroids (TCS) met the co-primary endpoints and all secondary endpoints in AD Up, the third pivotal Phase 3 study of Rinvoq in atopic dermatitis.

21. AstraZeneca announced on July 29 that its SGLT-2 inhibitor: dapagliflozin met all its primary and secondary endpoints in a phase 3 trial for patients with chronic kidney disease (CKD).
22. AbbVie announced on July 30 that the Phase 3 ADVANCE trial evaluating the investigational medicine atogepant, an orally administered CGRP receptor antagonist, met its primary endpoint of statistically significantly greater reduction in mean monthly migraine days, compared to placebo, for all doses across the 12-week treatment period.
23. The New England Journal of Medicine published the latest results from the Phase 2b trial for nirsevimab, a respiratory syncytial virus (RSV) monoclonal antibody developed by Sanofi Pasteur and AstraZeneca, on July 30, which showed that nirsevimab significantly reduced the cases of medically attended lower respiratory tract infections caused by RSV through the full RSV season.
Read More:
Pharmaceutical News of the Week | CPhI.CN - Performance
About the Author:
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

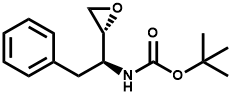
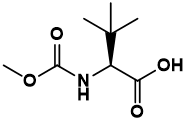


















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








