PharmaSources/zhulikou431June 24, 2020
Tag: inhalation formulations , respiratory disease , Chinese Market
Inhalation formulations are a dosage form with high technical barriers, with relatively few pharmaceutical enterprises entering this field at the beginning. More enterprises have started to make overall plan of inhalation formulations or entering this field by breaking through key varieties, with the worsening of environmental pollution and the increase of respiratory diseases in China.

Inhalation formulations are a drug dosage form where the drug is dissolved or dispersed in a suitable medium, directly enters the respiratory tract in the form of vapor or aerosol via a specific drug delivery device and is then delivered to the lungs to produce local or systemic therapeutic effects. They rapidly and directly improve the efficacy, with the adverse drug reactions reduced while the dosage is reduced, and have become the preferred therapeutic drugs for bronchial asthma, chronic obstructive pulmonary disease (COPD), and other respiratory diseases and also commonly used in the otorhinolaryngology department, surgery (inhalation anesthetics), and cardiology department.
In terms of different devices used, inhalation formulations are generally divided into MDI (metered-dose inhaler), DPI (dry powder inhaler), nebulizer solution (NEB), and inhalation spray, etc. This article focuses on the development and competition situation of inhalation formulations in the Chinese market taking the inhalation formulations of the respiratory system as an example.
Respiratory diseases are the second most common diseases in China, second only to cardiovascular and cerebrovascular diseases. Respiratory diseases are divided into acute and chronic respiratory diseases; wherein, chronic respiratory diseases include asthma and COPD, etc., accounting for 1/3 of the total, being the third major common chronic diseases after diabetes and cardiovascular diseases, and chronic respiratory diseases are also the third leading cause of death in China and need to be taken very seriously. Global respiratory disease medications are chemical inhalation preparations, the dosage forms are mainly aerosols and powder aerosols, with the drugs mainly used including ICS (budesonide and fluticasone, etc.) and bronchodilators (represented by salmeterol, formoterol, salbutamol, and tiotropium bromide) and compound formulations, etc. (see Table 2 for the details).
See Table 1 for the specific classification:
Table 1 Classification of Common Respiratory System Drugs
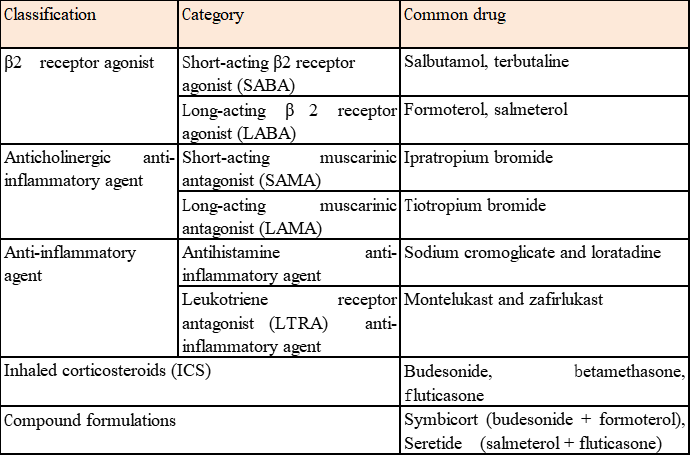
Table 2 Main Varieties of Global Respiratory System Drugs
Technical barriers to inhalation formulations
1. R&D barriers: Inhalation formulations are a combination of multiple technologies such as pharmacology, inhalation kinetics, particle dynamics, fluid mechanics, surface science and inhaler design and processing, etc., with very high R&D difficulties. Most inhalation formulations are also special formulations combining drugs and devices, with high requirements for drug-device linkage. As a result, appropriate inhalation devices are the core of R&D, and drug-device combination methods need to be sufficiently reasonable; furthermore, from the perspectives of process and prescription, particle size, dispersion state of the raw and auxiliary materials, ratio and combination method with the carrier, and moisture and ambient humidity control, etc. are key factors affecting the efficacy of drugs. Also, for MDIs, it’s necessary to make sure that the dose released by the drug delivery device every time is relatively stable. From the perspective of cost, sterile equipment used during the production and equipment used for production testing have high costs, which is also one of the factors restricting most generic drug enterprises.
2- Clinical trial and approval barriers: Clinical BE tests for inhalation formulations are complex. Take the FDA for instance, the FDA’s requirements for approving generic drugs of inhalation formulation include similar prescription and device to the original drug, equivalent systemic test, equivalent in vitro exposure PK, and same clinical efficacy; only when the four are met at the same time can the bioequivalence (BE) be established and the drug and device can be combined for application. However, there had been no mature BE review principles for inhalation formulations in China. The CDE issued the Research Guidelines for the Pharmacy and Human Bioequivalence of Generic Drugs of Orally Inhaled Drug Products (Draft for Comment) on Aug. 2, 2019, which proposes that in the human bioequivalence research evaluation, generic inhalation formulations generally need to be evaluated for human bioequivalence (consistency between the generic drugs and original drugs) through pharmacokinetic study (PK-BE study) and pharmacodynamic study (PD-BE study) or clinical endpoint study on the premise of consistent in vitro pharmaceutical quality; if only PK-BE study is used to evaluate the human bioequivalence, a linear relationship between the pharmacokinetics and local drug delivery equivalence of the product needs to be further proved.
3. Patent barriers: Foreign original products have not only conducted core patent layout in terms of formulation and device, but also conducted related patent layout in terms of excipient, etc., for example, GlaxoSmithKline (GSK) has multiple patents in its core products Seretide and Flixotide, with detailed patent protection in excipient selection and application, drug delivery device’s medicament pack, aerosol container valve, dispenser with a dose counter and dose indicator thereof, actuation indicator for the dispensing device, and other core product structures.
Foreign pharmaceutical enterprises and their products
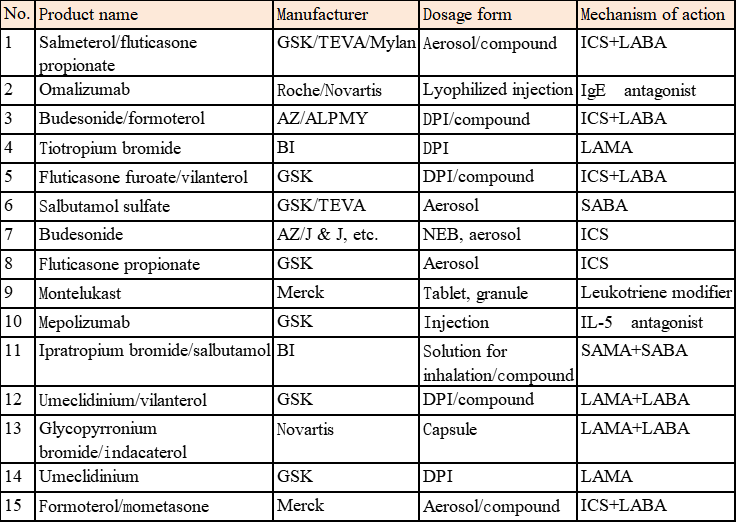
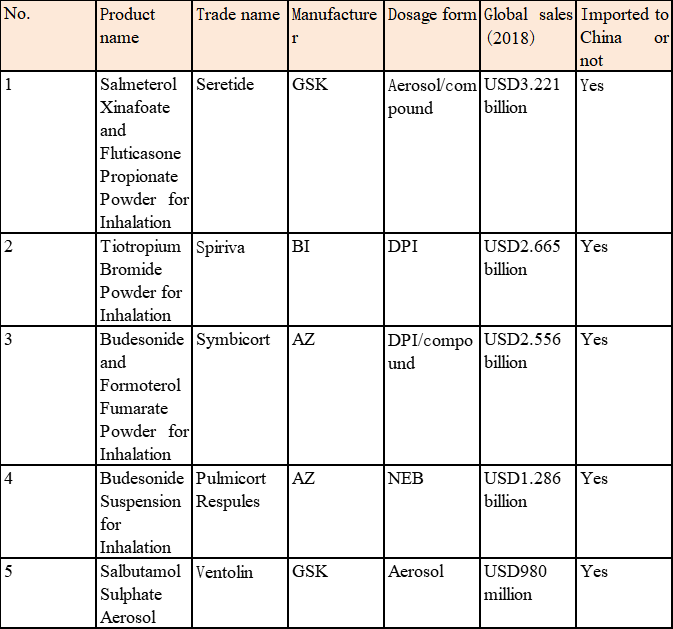
The three multinational pharmaceutical enterprises: AstraZeneca (AZ), GSK, and Boehringer Ingelheim (BI) are the three leading enterprises in global inhalation formulations and basically monopolize the blockbuster products of inhalation formulations in the respiratory system field in the world (Table 3).
In China, more than 90% of the related market has long been occupied by AZ, GSK, and BI, and there are few companies and products that can compete with them.
Table 3 Blockbuster Products of Global Inhalation Formulations
AZ
As a world-leading pharmaceutical enterprise, AZ has production bases in 17 countries and core products in six therapeutic areas including oncology, digestive system, respiratory system, anesthesia, cardiovascular system, and central nervous system. It has several blockbuster inhalation products in the respiratory system field registered in China: Budesonide and Formoterol Fumarate Powder for Inhalation (Symbicort), budesonide (Pulmicort Turbuhaler, Pulmicort, Pulmicort Respules), Terbutaline Sulphate Solution for Nebulization (Bricanyl), and Formoterol Fumarate Powder for Inhalation (Oxis Turbuhaler), etc., accounting for more than 40% market share in China.
GSK
As one of the large multinational pharmaceutical enterprises, GSK is strong in the four medical areas of anti-infection, central nervous system, respiratory system, and gastrointestinal tract/metabolism, and it also ranks top in the industry in terms of vaccine field and oncology drugs.
Inhalation formulation varieties registered by GSK in China mainly include salmeterol/fluticasone (Seretide) with annual sales of more than RMB1 billion and Salbutamol Sulphate Aerosol (Ventolin), Fluticasone Propionate Inhaled Aerosol/Fluticasone Propionate Nebuliser Suspension (Flixotide), Umeclidinium Bromide and Vilanterol Trifenatate Powder for Inhalation (ANORO) and Fluticasone Furoate, Umeclidinium Bromide and Vilanterol Trifenatate Powder for Inhalation (Trelegy Ellipta) with annual sales of more than RMB100 million, etc.
BI
BI is the world’s largest private pharmaceutical enterprise, mainly researching fields including immune and respiratory diseases, cardiovascular and metabolic diseases, central nervous system diseases, and oncology, etc.
Inhalation formulation varieties registered by BI in China mainly include Ipratropium Bromide Solution for Inhalation/Ipratropium Bromide Aerosol (Atrovent), Tiotropium Bromide Powder for Inhalation (Spiriva), Compound Ipratropium Bromide Solution for Inhalation (ipratropium bromide/salbutamol (Combivent)), Tiotropium Bromide and Olodaterol Hydrochloride Inhalation Spray (Spiolto), and Olodaterol Inhalation Spray, etc.
The above shows the industrial layout of inhalation formulations of international well-known enterprises in the Chinese market, from which we can see that international long-established enterprises have solid strength in the field. Then, what are the status quo and future competitive advantages of the Chinese pharmaceutical industry in this segment? Please stay tuned.
Note: This article does not constitute any value judgement or investment advice.
References:
1. NMPA website
2. Insight database
3. db.yaozhi.com
4. CDE website information
5. Joincare, CF PharmTech, Chiatai Tianqing and Hengrui Medicine websites
6. Chinese Pharmacopoeia Commission website
The Layout of Inhalation Formulations in the Chinese Market (II)
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


