PharmaSources/DopineJune 15, 2020
Tag: TRASTUZUMAB , biosimilar , Sunshine Guojian , Henlius
Breast cancer is the most common malignant tumor in women. According to statistics, there are about 270,000 new cases of female patients with breast cancer in China every year, of which about 1/4 are HER2 (human epidermal growth factor receptor 2)-positive. HER2-positive breast cancer has a high degree of malignancy and fast progression. Trastuzumab is the standard first-line regimen for adjuvant, neoadjuvant and metastatic cancer treatment of HER2-positive breast cancer.
The original trastuzumab is a HER2-targeted humanized monoclonal antibody developed by Roche. It can, by binding to the HER2 receptor, precisely treat patients with HER2-amplified breast cancer to reduce the postoperative recurrence of breast cancer and is thus known as the life-saving drug of breast cancer patients. The drug was approved for marketing in the U.S. in 1998 with the trade name Herceptin and approved in the EU in 2000, in Japan in 2001, and in China in 2002 (with the trade name: Herceptin). It’s worth mentioning that this original drug was listed in the WHO’s Model List of Essential Medicines in 2015.
Herceptin has quickly become Roche’s flagship product since marketed, with sales ranking among the top ten global bio pharma products by sales for years. However, Herceptin’s sales have started to decline since 2018, which is because of the impacts of biosimilars.
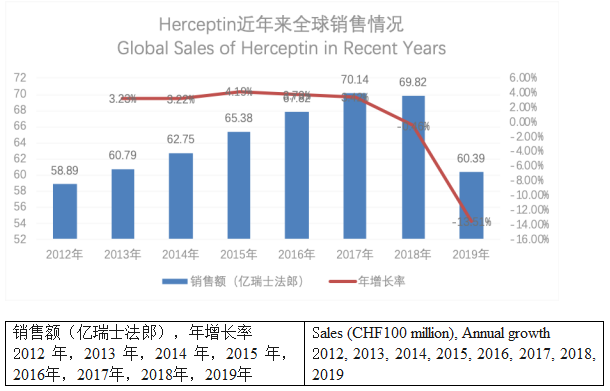
It is learned that Herceptin’s patents in Europe and Japan expired in 2014 and its patent in the U.S. expired in 2015. The FDA and EMA have so far approved many Herceptin biosimilars as detailed in the table below.
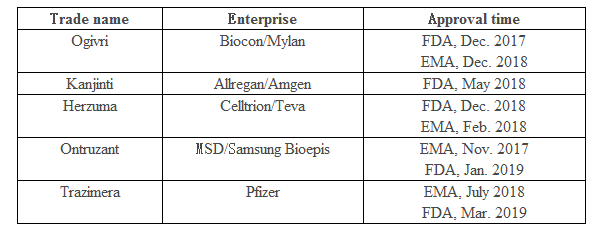
In addition to the European and U.S. markets, Herceptin biosimilar co-developed by Biocon/Mylan was approved by the Indian drug regulator early in Nov. 2013, and South Korean Celltrion’s biosimilar Herzuma was approved by the Ministry of Food and Drug Safety (MFDS) of South Korea in 2014.
It’s worth mentioning that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recently released a positive review opinion, recommending the approval of the marketing authorisation application (MAA) of Henlius’ HLX02 (Trastuzumab Injection) for treating HER2-positive early breast cancer, HER2-positive metastatic Breast cancer, and previously untreated HER2-positive metastatic adenocarcinoma of the stomach or gastro-oesophageal junction. The European Commission (EC) is expected to make the final decision in the next 2-3 months with reference to the CHMP’s review opinion. If approved, Henlius’ HLX02 will become the first “Chinese nationality” monoclonal antibody biosimilar that enters the European market.
Instead of awaiting its doom, Roche has developed Perjeta and Kadcyla in advance to respond to the impacts of Herceptin biosimilars; wherein, Perjeta, i.e., pertuzumab, is a monoclonal antibody that targets HER2, with the same mechanism of action as Herceptin, but it has a different binding site (Herceptin specifically binds to the 4th functional domain (D4) extracellularly of HER2 protein to block signal transduction and mediate cellular immunity to kill tumors; while pertuzumab specifically binds to the 2nd functional domain (D2) extracellularly of HER2 protein to block HER2 dimerization and inhibit HER2 activation). The combination of the two can more completely block the HER2 signaling pathway to thus stop the growth and survival of cancer cells. Kadcyla (T-DM1, Trastuzumab Emtansine) is an antibody-drug conjugate that can bind to tubulin’s cytotoxic compound Maytensine to achieve covalent binding to trastuzumab via thioether bond; and through trastuzumab’s affinity to HER2, T-DM1 will be taken within by tumor cells after binding to HER2 protein, the lysosome inside the cells will degrade trastuzumab protein, and the toxin Maytensine not degraded will bind to tubulin to kill tumor cells.
Furthermore, Roche has also launched the subcutaneous injection dosage form of Herceptin: Herceptin Hylecta. It is a ready-to-use formulation that can be administered within 2-5 minutes, with better patient compliance than the traditional intravenous infusion dosage form.
The CDE issued the Clinical Study Design and Review Considerations of Biosimilars to Trastuzumab Injection in China in Nov. 2017 to solicit public opinions, which refines the imitation strategy in combination with the characteristics of the variety and encourages more Chinese enterprises to actively develop Herceptin biosimilars.
However, there is no trastuzumab biosimilar approved so far in China. Roche, to retain its market share, has adopted the price reduction strategy. Herceptin’s price declined from RMB24,500 to RMB7,600 per piece in 2017, and it successfully entered the national reimbursement drug list (NRDL) of China through a nearly 70% decline; it successfully stayed in the NRDL in 2019 through continuous price reduction. Roche’s pertuzumab also entered the NRDL in 2019 through price reduction.
According to the Insight database, both Sunshine Guojian and Henlius have filed the marketing application for trastuzumab biosimilar. And according to the review timeline of the related acceptance number, Sunshine Guojian is slightly ahead of Henlius and expected to be the first in obtaining the marketing approval for trastuzumab biosimilar. In fact, Sunshine Guojian has filed the marketing application for trastuzumab-302H early in Apr. 2011, however, it withdrew the application in May 2016 because of the later inclusion in the List Subject to Self-inspection and Checking of Drug Clinical Trial Data.
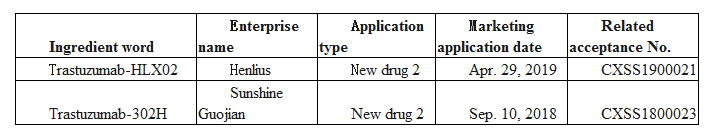
According to the Insight database, both Sunshine Guojian and Henlius have filed the marketing application for trastuzumab biosimilar. And according to the review timeline of the related acceptance number, Sunshine Guojian is slightly ahead of Henlius and expected to be the first in obtaining the marketing approval for trastuzumab biosimilar. In fact, Sunshine Guojian has filed the marketing application for trastuzumab-302H early in Apr. 2011, however, it withdrew the application in May 2016 because of the later inclusion in the List Subject to Self-inspection and Checking of Drug Clinical Trial Data.
However, it is worrying that the generic name used in the “302H” project description in Sunshine Guojian’s prospectus is “inetetamab”, which may affect its direct entry into the NRDL after it is approved.
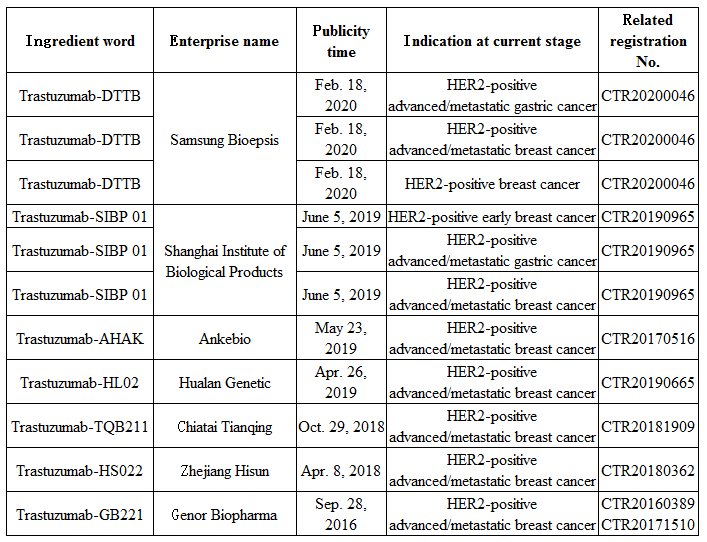
Furthermore, according to Insight database, enterprises including Samsung Bioepsis, Shanghai Institute of Biological Products, Ankebio, Hualan Genetic, Chiatai Tianqing, and Zhejiang Hisun, etc. are conducting related phase III clinical trials. As a result, the trastuzumab market in China is expected to become increasingly fierce in the future.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


