PharmaSources/LaoxueMay 21, 2020
Tag: ranitidine , Helicobacter pylori infection , CHMP , NDMA , FDA
The Committee for Medicinal Products for Human Use (CHMP) under the EMA has recommended the suspension of all ranitidine drugs in the EU on Apr. 30.
This is another notification issued by a foreign organization on the suspension of ranitidine following the U.S. FDA’s requirement on Apr. 1 that pharmaceutical enterprises shall immediately remove all ranitidine drugs from the market.
Ranitidine has many indications overseas, including the gastrointestinal tract and esophagitis treatment, etc.
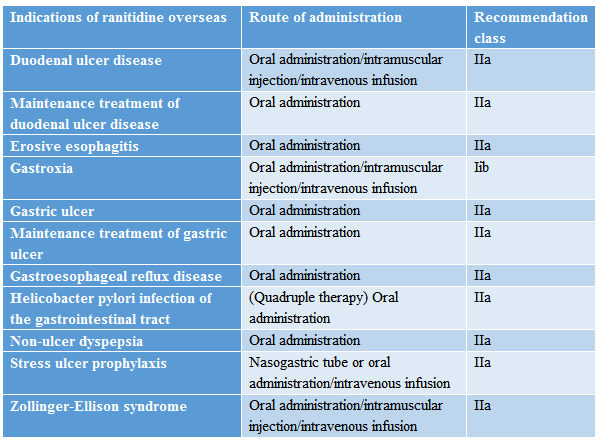
Source: Medicine Helper APP
Wherein, the quadruple therapy used overseas for Helicobacter pylori infection of the gastrointestinal tract includes ranitidine, metronidazole, basic bismuth subsalicylate, and tetracycline hydrochloride, unlike that in China.
The method used in China is selecting the antimicrobial agents (such as amoxicillin, tetracycline, and furazolidone) that are known to have low drug resistance rates in combination with bismuth (i.e., bismuth-containing quadruple therapy) and increasing the dose of metronidazole. From this aspect alone, for patients with Helicobacter pylori infection of the same number in China and overseas, the demand for ranitidine overseas will be far higher than that in China. Furthermore, there is also a certain number of off-label uses.
Uses outside the FDA Package Insert (Adults)
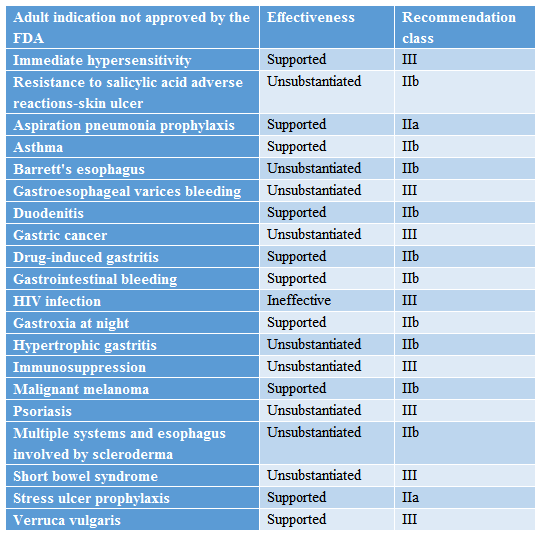
Source: Medicine Helper APP
The reason for the suspension given by the CHMP is the same as that of the FDA: the potential carcinogenicity of the impurity NDMA in ranitidine. The valsartan event caused due to NMDA aroused wide concern last year, and API enterprises including Huahai Pharmaceutical, etc. were affected.
According to queries on the website of the National Medical Products Administration of China (NMPA), ranitidine, an important gastrointestinal drug, has 579 formulations in China, including injection, tablet, effervescent tablet and capsule dosage forms.
And there are 16 related API enterprises in China.
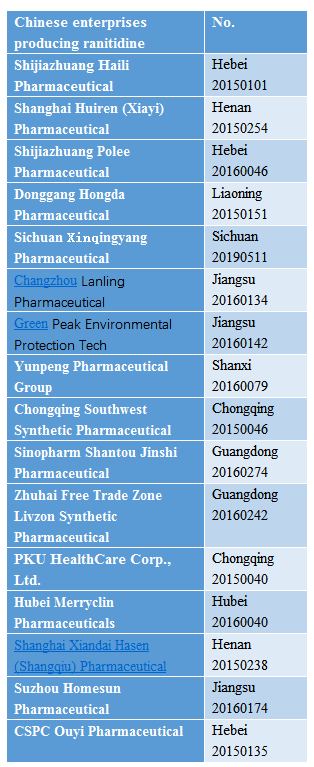
Source: NMPA website
Based on previous experience, there is a pattern that many pharmaceutical products are banned first abroad and then in China. For example, Analgin was officially banned by the U.S. FDA and removed from the U.S. market in 1977, and the clinical applications of many dosage forms were halted. Thereafter, about 30 countries including Japan, Australia, and Iran, etc. have explicitly banned or restricted the use of Analgin in succession, and China announced the cancellation of Analgin injection and other Analgin varieties on Mar. 17, 2020.
Whether ranitidine will also go on such a path is unknown, however, there is something different: NDMA is an impurity instead of a substance necessarily present in ranitidine. Whether the impurity can be eliminated by optimizing the process is a problem for enterprises. However, the removal of this impurity is very difficult from the current view, otherwise, foreign enterprises would not have easily agreed to the banning requirement.
And, as old pharmaceutical bio based products, ranitidine’s “dividend period” should also be approaching the end. It is possible that related enterprises will take the initiative to remove ranitidine products from the market considering the decline in profits and the risks. Furthermore, the comparison between any pharmaceutical product’s therapeutic effects and risks is related to whether it is irreplaceable. The removal of ranitidine suggests that other drugs can substitute it, such as omeprazole. If there were no other substitutes, ranitidine would not have been banned even if it had carcinogenic risks. A chain reaction may be caused in China within a period. Therefore, related enterprises should be prepared now, to avoid the sudden cancellation similar to that of Analgin.
References:
Fifth Chinese National Consensus Report on the Management of Helicobacter pylori Infection
Medicine Helper APP
NMPA website
https://www.ema.europa.eu/en/news/suspension-ranitidine-medicines-eu
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of PharmaSources.com,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@imsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


