PharmaSources/zhulikou431November 14, 2019
Tag: Injection , SMA , Nusinersen , Imported Drug
Nusinersen Injection, the world’s first SMA (Spinal Muscular Atrophy) precision targeted therapy, was completed the intrathecal injection to the first SMA patients in China on Oct. 10, 2019, marking a new milestone of SMA treatment.
SMA is a rare genetic neuromuscular disease, with an incidence of about 1/6,000-1/10,000 in newborns. SMA is divided into SMA-I, II, III, and IV according to the onset age and motor milestone achieved. Most SMA-I infantile patients cannot survive to 2 years old if they are not treated. The disease did not have any therapy until the marketing of Nusinersen Injection. Therapeutic measures for SMA in China and worldwide were only limited to adjuvant therapies such as respiratory support, nutritional support, and orthopedics.
Nusinersen Injection (registered trade name: SPINRAZA in the U.S. and the EU) is developed by Biogen Idec Ltd and was first approved in the U.S. on Dec. 23, 2016, being the world’s first SMA precision targeted therapy. The drug has so far been approved in the EU, Brazil, Japan, South Korea, and Canada, etc. for treating SMA. Nusinersen Injection was officially approved by the National Medical Products Administration of China (NMPA) on Feb. 22, 2019, to treat 5q SMA and become the first SMA drug in China.
Nusinersen (Spinraza) is an antisense oligonucleotide that can alter SMN2 splicing and increase the production of full-length SMN protein, being a gene therapy drug. Nusinersen Injection can directly deliver the drug to the cerebrospinal fluid around the spinal cord through intrathecal injection, to improve the motor function, increase survival and change SMA progress. The drug won the "Best Biotechnology Product" in the 2018 International Prix Galien awarded in Nov. 2018.
Nusinersen Injection (Spinraza) is quite expensive in the U.S.: it is priced at USD125,000/injection and requires 6 injections in the first year, costing about USD750,000, and the cost for the second year is halved to USD375,000. The unit selling price of the drug is RMB697,000 in China, which has set a new record of pharmaceutical selling prices in China, and patients have to pay at their own expense. We look forward to that the SMA drug could be included in China’s national or local medical insurance drug catalog soon, to help SMA patients’ families solve the burden of treatment and make guaranteeing the drug access of rare disease patients become a reality.
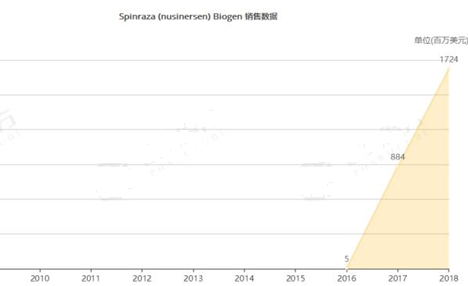
Figure: Sales Data of Spinraza (source: Pharmcube, NextPharma)
Nusinersen Injection (Spinraza) has obtained the Orphan Drug Designation both in the U.S. and the EU. SMA was listed in the First List of Rare Diseases jointly issued by departments including the National Health Commission of China (NHC), etc. in May 2018. The entire approval cycle of Spinraza was less than 6 months (173 days) in China, and its approval date was also only 2 years and 2 months (794 days) from its first approval in the U.S. Such fast approval speed was thanks to China’s series of policies for rare diseases and overseas new drugs catering to clinical urgent needs:
In Dec. 2017, the former China Food and Drug Administration issued the Opinions of the China Food and Drug Administration on Encouraging Priority Review and Approval for Drug Innovation, clearly proposing to add rare disease drugs to the scope of priority review.
In May 2018, the NMPA and NHC issued the Announcement on Matters concerning the Optimization of Drug Registration Review and Approval, clearly proposing that for the pharmaceutical products already marketed overseas for prevention and treatment of diseases that are seriously life-threatening and have no effective treatment means, and rare diseases, if applicants for registration of these imported pharmaceutical products have confirmed after study there is no any ethnic difference, they can directly apply for drug marketing registration via submitting the clinical trial data obtained overseas.
The executive meeting of the State Council of China on June 20, 2018 proposed to orderly accelerate the marketing approval of overseas marketed new drugs in China and simplify the marketing requirements for some pharmaceutical products that treat rare diseases and prevent and treat seriously life-threatening diseases.
The NMPA issued the Technical Guidelines for Accepting Overseas Clinical Trial Data of Drugs in July 2018, to support the entry of some imported rare disease pharmaceutical products that meet relevant conditions to China without the need for doing clinical trials in China.
The CDE issued the first list of 48 overseas new drugs catering to clinical urgent needs on Aug. 8, 2018.
Nusinersen Injection was included in the priority review and approval procedures by the NMPA as an overseas marketed, clinically imperative new drug for rare disease treatment in Sep. 2018.
On Oct. 31, 2018, the NMPA and the NHC issued the Work Procedures for the Review and Approval of Overseas New Drugs Catering to Clinical Urgent Needs and application data requirements, to set up a special channel for the review and approval of such new drugs and undertake to complete the review of rare disease pharmaceutical products and other overseas new drugs separately within 3 months and 6 months.
The CDE issued the second list of 30 overseas new drugs catering to clinical urgent needs on Mar. 28, 2019.
China continues to pay attention to imported drugs catering to clinical urgent needs and rare disease drugs. According to incomplete statistics, a total of 102 (involving 59 products) imported pharmaceutical products was approved in 2018, and 25 products thereof (see Table 1 Imported Drugs Approved through Priority Review in 2018) were approved for marketing through priority review, most of which were innovative drugs with significant therapeutic advantages, pediatric drugs, and rare disease drugs. Wherein, MSD’s 9-valent HPV vaccine (HPV vaccine) approved for marketing in China is called "the pharmaceutical product fastest approved in history", which only took 9 days from the application filing to the approval for marketing. The situation was highly praised by the public. Furthermore, the imported drug registration application of Roche’s alectinib capsules was approved in Aug. 2018, to treat ALK-positive advanced non-small cell lung cancer as a first-line therapy, which is called "the new drug with the shortest marketing lag" as it was marketed in China almost synchronously with the EU; the drug was approved in the U.S. and EU separately in Nov. and Dec. 2017.
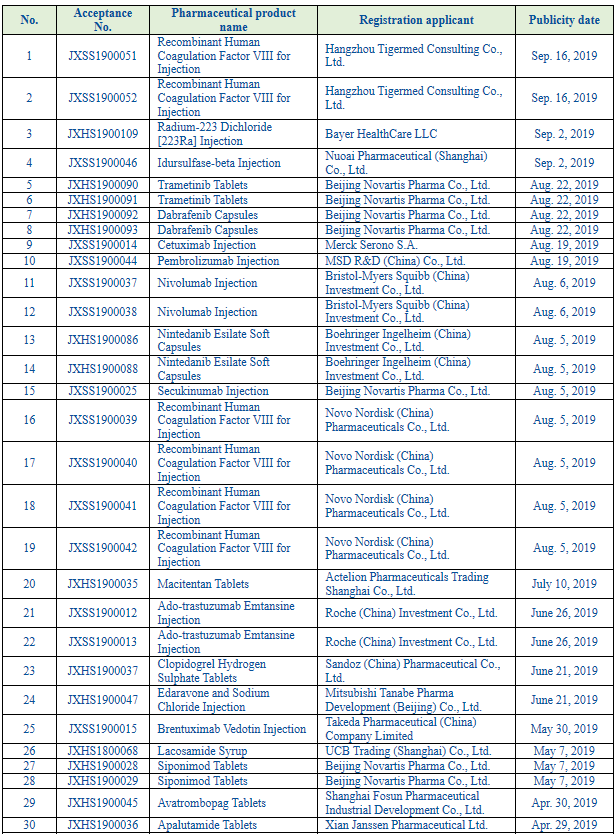
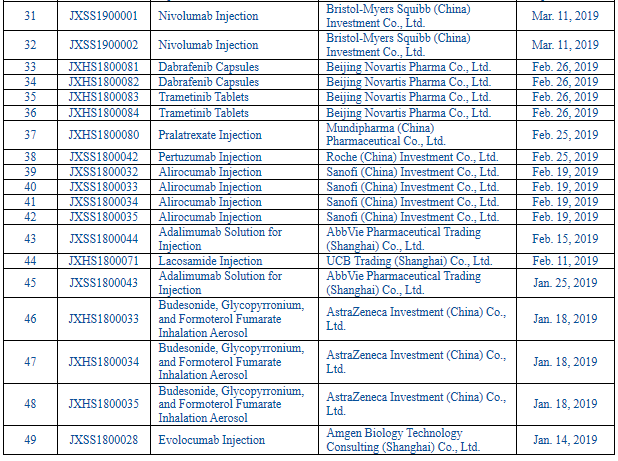
And, according to the priority review public data of the CDE, there have been 950 varieties included in the list of varieties subject to priority review since 2016, including 306 imported pharmaceutical products and 49 in 2019 (see Table 2 List of Imported Pharmaceutical Products Included in the Priority Review as Publicized by the CDE in 2019 for details).
Table 1 Imported Drugs Approved through Priority Review in 2018
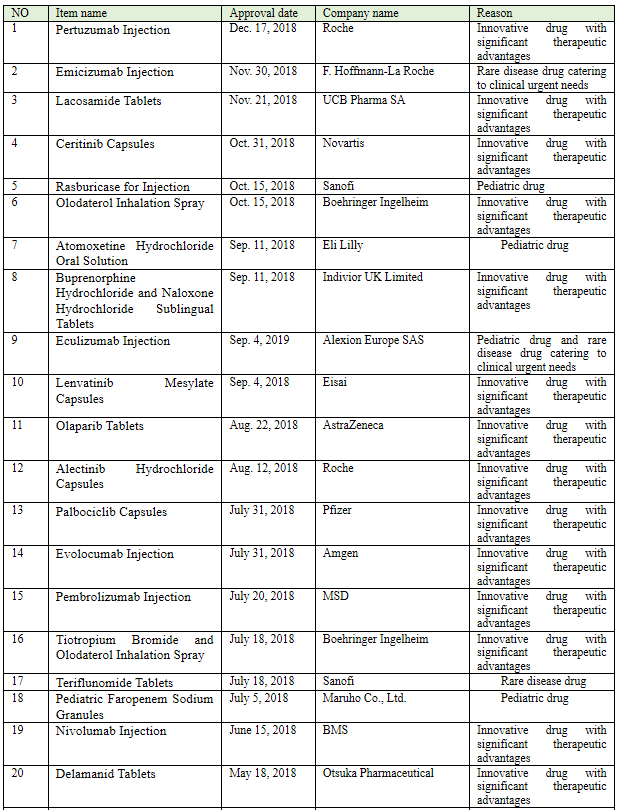
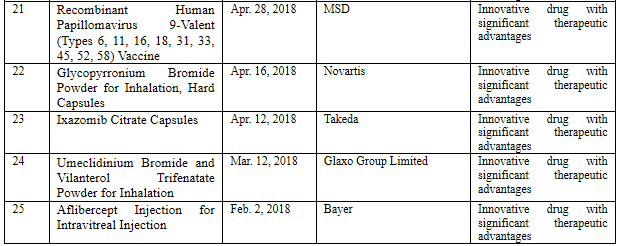
Table 2 List of Imported Pharmaceutical Products Included in the Priority Review as Publicized by the CDE in 2019
Nusinersen Injection is expensive, however, it brings the hope of life to patients and their families in the face of disease pain and death threats. It’s hoped that the NMPA and CDE could continue to optimize the pharmaceutical product review and approval process by upholding the Scientific Outlook on Development, to promote the timely marketing of more Chinese and overseas excellent drugs to earlier and better serve patients.
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


