PharmaSources/CaicaiJuly 24, 2019
Tag: china , multiple sclerosis , Blockbuster , Fingolimod
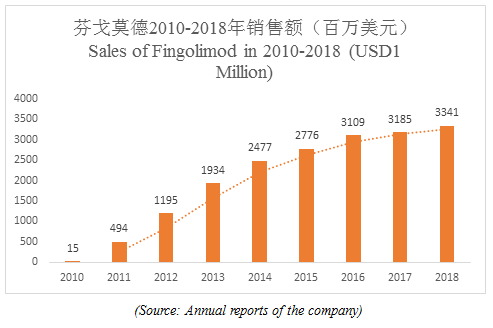
Big news! Novartis’ Fingolimod Hydrochloride (trade name: Gilenya) will be marketed in China soon. As the world’s first oral drug for multiple sclerosis (MS), fingolimod achieved global sales of USD3.341 billion in 2018, making it a well-deserved "blockbuster drug".
Related News: A Review of MS drugs in China

A blockbuster in the MS area
—Overview
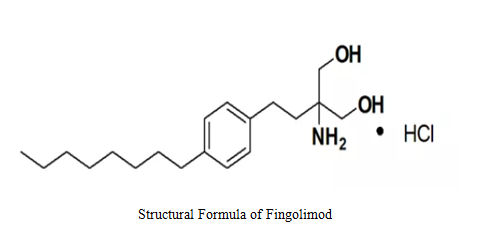
Fingolimod is a new immunosuppressant developed by Novartis, one of the largest pharmaceutical companies and medical product suppliers in the world.
The drug was originally a compound with strong immunosuppressive activity separated from the fungus Cordyceps sinclairii and its "close relative" Cordyceps sinensis, belonging to sphingosine-1-phosphate receptor (S1PR1/S1PR3/S1PR4/S1PR5) regulator. Fingolimod is a prodrug and the substrate of sphingosine kinase 2 (SPHK2); it is phosphorylated by SPHK2 in vivo to generate the active phosphate ester (S’)-FTY720-P, being the full agonist of S1P1,3,4,5; (S’)-FTY720-P can play the role of functional antagonism and inhibiting S1P1-mediated lymphocyte migration by activating S1P1 receptor and inducing receptor invagination, to help T lymphocytes stay within lymph nodes and trigger peripheral lymphocytes to play the "homing function" to thus produce the immunosuppressive action.
—Approved indications
It has been approved in the U.S., EU, and Japan and filed the marketing application to the NMPA in Apr. 2019.
The indications approved include MS and relapsing-remitting MS (RRMS).
Approval time | Indication |
Sep. 2010, May 2018 | Approved by the FDA for the treatment of MS; Treatment of relapsing MS in children and adolescents aged 10 years and older (the first drug for treating MS in children) |
Mar. 2011, Jul. 2014 | Approved by EMA for the treatment of RRMS; Treatment of RRMS in patients not responding to interferon-beta injection treatment |
Sep. 2011 | Approved by the PDMA for the treatment of MS |
(Sorted out according to public data)
—Adverse reactions
According to the warning of FDA on Nov. 20, 2018, when fingolimod is stopped after MS patients receive it, the disease can become much worse than before the medicine was started and the worsening can even result in permanent disability. As a result, the FDA suggested that patients should be informed about the potential risk of a severe increase in disability before starting treatment and when fingolimod is stopped, patients should be timely observed for adverse reactions.
—Patent layout
The global patent applications of fingolimod have exceeded 100. The original company has applied for a series of patents to protect fingolimod, for example, the parent patent US-5604229 was granted a 5-year extension in Dec. 2010 and expired in Feb. 2019; the compound patent US-08324283 has been granted in most major markets and will expire in 2026; the new use patent (US-09187405) was granted in Jan. 2016 and will expire in 2027.
—Global sales
Sales of fingolimod have continued to grow since it was marketed in 2010, with global sales reaching USD3.341 billion in 2018, however, the global sales of fingolimod may slow down in the future due to the exposure of many adverse reactions.
Related News:
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


