PharmaSources/CaicaiJuly 02, 2019
Tag: Blockbuster , Sino Biopharmaceutical , giants
Sino Biopharmaceutical is one of the 3 traditional giants in China. Let’s see Sino Biopharmaceutical’s R&D pipelines and blockbuster products to be marketed in the next 3 years below.

Leading in R&D expenditure
Sino Biopharmaceutical, a company established in 2000, has been moving ahead steadily and surely, and its position as one of the 3 traditional giants in China (Hengrui, CSPC, and Sino Biopharmaceutical) has been firm, with the famous Chiatai Tianqing being a subsidy thereunder.
Sino Biopharmaceutical achieved revenue of RMB20.89 billion in 2018, growing by 41.0% year on year; profit attributable to owners of the parent amounted to RMB9.05 billion, growing by 316.7% year on year; R&D expenditure amounted to RMB2.091 billion, growing by 31.0% year on year, accounting for 10.0% of the revenue. Its R&D expenditure exceeded RMB2 billion for the first time, with the growth significantly higher than that in 2017 (16.6%), and the proportion of its R&D expenditure in revenue reached 10% which was higher than many pharmaceutical enterprises, showing the great importance attached by Sino Biopharmaceutical to the R&D.
R&D pipelines: "strong ones stay strong, weak ones become strong"
From the perspective of indication, Sino Biopharmaceutical’s R&D pipelines will continue to keep ahead in next years in the liver disease and cardiovascular and cerebrovascular disease areas which have been traditionally its strong points, and have been actively seizing territory in emerging therapeutic areas such as tumor, respiratory and blood diseases; from the perspective of drug variety, in terms of generic drugs, Sino Biopharmaceutical’s position as the "king of first generics" is still as firm as a rock; in terms of innovative drugs, Sino Biopharmaceutical will have more and more innovative drugs in the future beginning with anlotinib. In short, "strong ones stay strong, weak ones become strong".
Revenue and proportion of each indication of Sino Biopharmaceutical in 2018 are as follows:
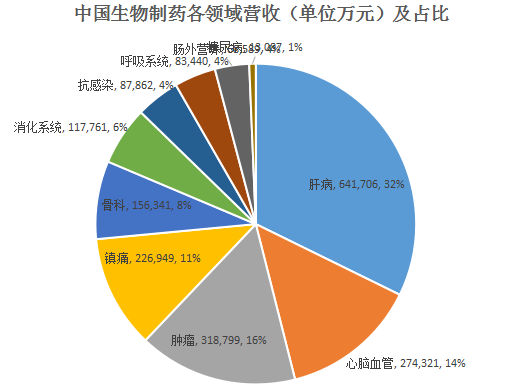
中国生物制药各领域营收(单位万元)及占比 | Revenue (in RMB10,000) and Proportion of Each Area of Sino Biopharmaceutical |
消化系统 | Digestive system medicines |
抗感染 | Anti-infectious medicines |
呼吸系统 | Respiratory system medicines |
肠外营养 | Parenteral nutritious medicines |
糖尿病 | Diabetes medicines |
骨科 | Orthopedic medicines |
镇痛 | Analgesic medicines |
肿瘤 | Oncology medicines |
肝病 | Hepatitis medicines |
心脑血管 | Cardio-cerebral medicines |
(Source: Annual Report of the company)
Sino Biopharmaceutical has now 467 products in development, including 199 oncology medicines, 53 cardio-cerebral medicines, 33 hepatitis medicines, 22 respiratory system medicines, and 26 diabetes medicines, etc. (Next, I’ll list blockbuster products to be marketed in the next 2-3 years due to the huge number of the products in development, and I’ll update readers in future).
In terms of generic drug, Sino Biopharmaceutical will have more than 20 generic drugs marketed in the next 2-3 years, wherein, the number of first generics will approach 15, making it the well-deserved "king of first generics"; in terms of innovative drug, it will have more indications approved successively in the future besides anlotinib marketed last year, and its anti-PD-L1 monoclonal antibody TQB2450 is expected to be marketed in 2021.
Next, key pharmaceutical products of each indication above will be separately analyzed in the order of the above indications.
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


