PharmaSources/MilingMay 05, 2019
Tag: SCY-078 , Superbug , drug tolerance , antifungals
The China Newsweek has recently reported on the infections of the "superbug"—Candida auris (C. auris) in China. According to Liao Wanqing, an academician of the Chinese Academy of Engineering and professor in the Dermatology Department of Changzheng Hospital, Second Military Medical University, "18 cases of clinical infection with the superbug have so far been confirmed in China." The infections of the superbug have been much concerned by the public due to the high fatality rate (more than 60%) thereof. The U.S. Centers for Disease Control and Prevention (CDC) has listed C. auris as an imminent threat, and the "superbug" control situations are also very severe in the UK, Japan, and India, etc. And as there is still no specific drug, more than 50% "superbug" infected people will die in 90 days. Such severe situations have raised very exigent requirements to the pharmaceutical practitioners.
I. About the "superbug"-C. auris
The scientific name of the "superbug" is C. auris which was first discovered in Japan in 2009 and has thereafter rapidly spread. Countries where the "superbug" has been reportedly separated and obtained include Canada and U.S. in North America, Colombia, Panama, and Venezuela in South America, Germany, France, Norway, Spain, UK, Switzerland, Russia, Belgium, and Austria in Europe, South Africa and Kenya in Africa, and India, Israel, Japan, Kuwait, Oman, Pakistan, South Korea, China, Malaysia, Saudi Arabia, and the United Arab Emirates in Asia. Wherein, Austria, Belgium, China, Malaysia, Norway, Russia, Switzerland, and the United Arab Emirates had all reported one case. This "superbug" has swept the globe.
In terms of the harm, C. auris can infect patients’ blood, lung, urethra, surface wound, and ear canal, etc. The external environmental factors that cause C. auris infections are similar to those that cause other pathogenic candida infections, with the risk factors including inserted catheters (urinary, central venous and arterial catheters), invasive medical procedures and equipment, use of breathing devices, long-term hospitalization, ICU (intensive care unit) patients, continuous treatment with broad-spectrum antifungals or antibiotics or treatment with immunosuppressants, and patients complicated with other diseases like diabetes and HIV. An understanding of the above is of some significance to the prevention of C. auris infection.
II. Drug susceptibility and resistance of the "superbug"
The medication standard of the minimum inhibitory concentration (MIC) of C. auris has still not been established. With reference to the medication standards of other candida, it turns out that almost all strains are highly resistant to fluconazole, more than half are resistant to voriconazole, 1/3 strains are resistant to amphotericin B (MIC≥2μg/mL), and a few strains are also resistant to echinocandins. Researchers have tested the susceptibility of 90 strains of C. auris to the common antifungals through the broth microdilution method recommended by the Clinical and Laboratory Standards Institute (CLSI), it turned out C. auris had high MIC90 values against fluconazole, amphotericin B, 5-fluorocytosine, and caspofungin, separately 64, 4, 8, and 1 μg/mL (Fig. I).
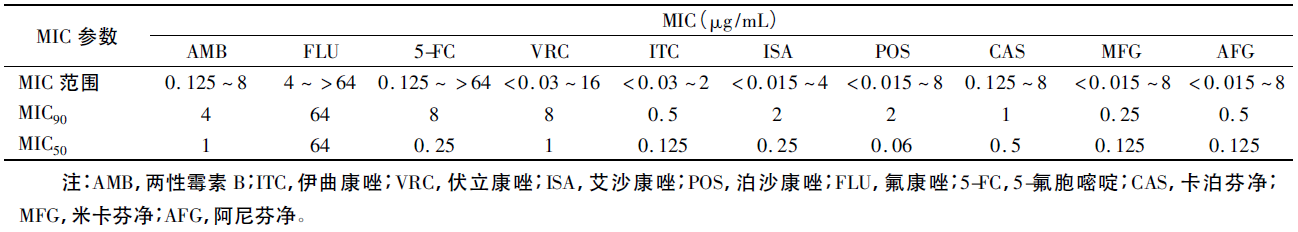
MIC参数 | MIC parameter |
MIC范围 | MIC range |
注:AMB,两性霉素B;ITC,伊曲康唑;VRC,伏立康唑;ISA,艾沙康唑;POS,泊沙康唑;FLU,氟康唑;5-FC,5-氟胞嘧啶;CAS,卡泊芬净;MFG,米卡芬净;AFG,阿尼芬净 | Note: AMB, amphotericin B; ITC, itraconazole; VRC, voriconazole; ISA, isavuconazole; POS, posaconazole; FLU, fluconazole; 5-FC, 5-fluorocytosine; CAS, caspofungin; MFG, micafungin; AFG, anidulafungin |
Fig. I In Vitro Susceptibility of 90 Strains of C. auris to Common Antifungals
According to my research, the mechanism of C. auris’ resistance to antifungals is still a mystery, and only some reports have pointed out that its drug resistance is caused by the rapid mutation induced under the antifungal selective pressure.
III. An embarrassing situation of the "superbug": No cure
The resistance of the "superbug" to main antifungals such as azoles, polyenes, and echinocandins has caused it much difficult to treat. Drug researchers have made great efforts in this regard. Fig. II shows the number of papers published by month on C. auris in journals between 2009 and 2018, according to which, the number of studies on the "superbug" has been growing in recent years.
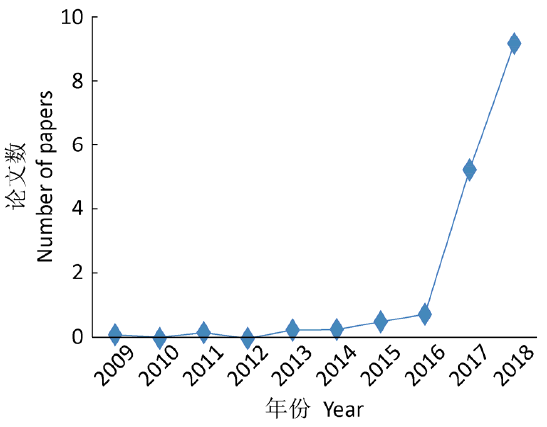
Fig. II Average Number of Papers Published by Month on C. auris in Journals between 2009 and 2018
However, unfortunately, it is apparent that patients infected by C. auris (most of them have less than 90 days to live after getting infected) cannot wait for the successful development of new drugs, and only those published papers are far from enough. Drugs reported by pharmaceutical enterprises that are effective against C. auris include VT-1598 under Viamet Pharmaceuticals, which is in the preclinical development, and SCY-078 under Merck, which is also in the clinical development stage (a clinical trial with 30 patients with C. auris has not been completed).
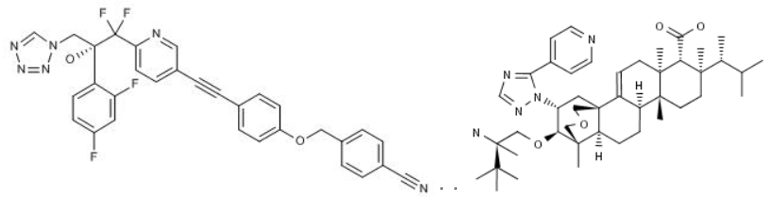
Fig. III Structural Formulas of VT-1598 and SCY-078
IV. Current countermeasures and prospects
The best countermeasure is prevention when there is still no specific drug. Relatively to the large number of outbreaks in the U.S., China can be said to have done a complete job in the prevention, however, further prevention can start from the source and pathogenesis, etc. of the "superbug", and it’s also necessary for infected people to receive corresponding treatment under the guidance of doctors.
The hope is that from the above analysis, studies on the "superbug" have been increasing, and although the progress of relevant drugs is slow, some drugs have entered the clinical stage, and SCY-078 has received the orphan drug designation by FDA; therefore, we can be sure that drugs against the "superbug" will be developed. And the gravity of the current situations has brought higher and more urgent requirements to pharmaceutical practitioners. They must work together to keep patients from waiting too long!
References:
Miling, Master of Pharmacy, majored in biopharmaceutical, has long been engaged in new drug research and development, focusing on the analysis of trends in the domestic and overseas drug market, and is good at the research and development of biological drugs and small molecule drugs.
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


