PharmaSources/1℃February 13, 2019
Tag: AbbVie , Humira , drug king
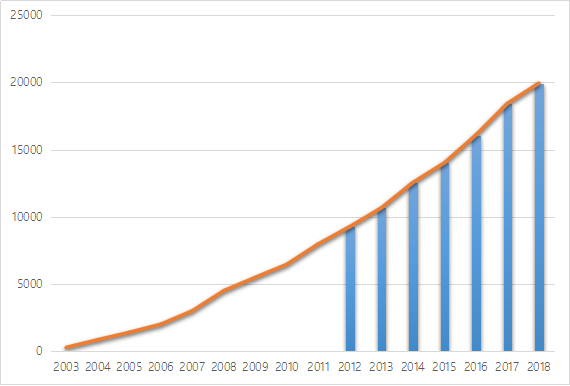
AbbVie released its 2018 financial report on Jan. 25, 2019, according to which, the 2018 sales of Humira reached USD19.936 billion, securing its position as the global drug king and topping for seven times in terms of sales!
Humira: AbbVie has reached settlements with many companies
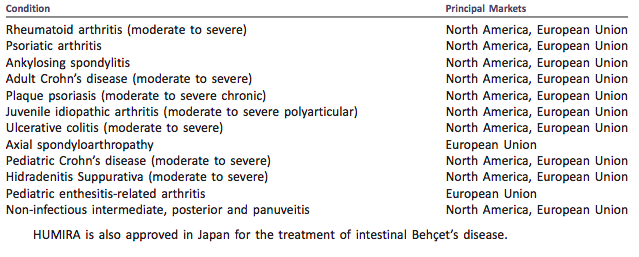
As the first fully human monoclonal antibody marketed, Humira (adalimumab) has been marketed in major regions of the world with a total of 14 indications, since marketed in 2002.
Marketed for 16 years, AbbVie’s Humira has gradually become a super-blockbuster and replaced Lipitor to top as the global drug king for 7 consecutive years, accumulatively bringing USD132.878 billion sales revenue to AbbVie.
Many manufacturers are eying Humira that props up AbbVie as a super-blockbuster. So far, AbbVie has reached patent settlements with 9 manufacturers (as shown in the following table). Humira will continue its myth for years, however, it will eventually be impacted by biosimilars and its sales will gradually decline.
Biosimilar patent settlements and expected marketing time
Trade name | Company | Stage | EMA marketing time | US marketing time | Patent settlement region |
Imraldi | MSD Samsung Bioepis | Approved for marketing | Not earlier than Oct. 16, 2018 | Not earlier than June 30, 2023 | U.S., most EU region, and other marketing regions |
Cyltezo | Boehringer Ingelheim | Approved for marketing | N/A | N/A | No settlement reached |
Halimatoz Hefiya Hyrimoz | Novartis | Approved for marketing | Not earlier than Oct. 16, 2018 | Not earlier than Sep. 30, 2023 | U.S., most EU region, and other marketing regions |
Amgevita | Amgen | Approved for marketing | Oct. 16, 2018 | Not earlier than Jan. 31, 2023 | U.S., most EU region, and other marketing regions |
Hulio | Kyowa Hakko Kirin; Mylan | Approved for marketing | Not earlier than Oct. 16, 2018 | Not earlier than July 31, 2023 | U.S., and other marketing regions, except EU |
| Merck KGaA ; Fresenius | Phase 3 | Available for sale upon marketing | Not earlier than Sep. 30, 2023 | U.S. and EU |
| Momenta | Phase 3 | Available for sale upon marketing | Not earlier than Nov. 20, 2023 | U.S. and EU |
| Pfizer | Phase 3 | Available for sale upon marketing | Not earlier than Nov. 20, 2023 | U.S. and EU |
| Coherus BioSciences | Phase 3 | N/A | Not earlier than Dec. 15, 2023 | U.S. |
Related:
Overview of bio-macromolecular drugs in the autoimmune disease field in China
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


