pharmaceutical-technologyJanuary 03, 2019
Tag: NASH , market forecast , 2026
Due to increasing rates of obesity and diabetes, non-alcoholic steatohepatitis (NASH), a chronic inflammatory liver disease, is becoming increasingly prevalent. Currently, there are no approved treatments for the disease, yet, GlobalData forecasts that the NASH market will grow at a Compound Annual Growth Rate (CAGR) of 63% across the US and the five major European markets (5EU: France, Germany, Italy, Spain and the UK), reaching $18.3B by 2026.
These figures imply that an attractive market exists for biopharma and biotech companies, as well as potential investors. This article will discuss this deal activity and what it reflects for the wider NASH space.
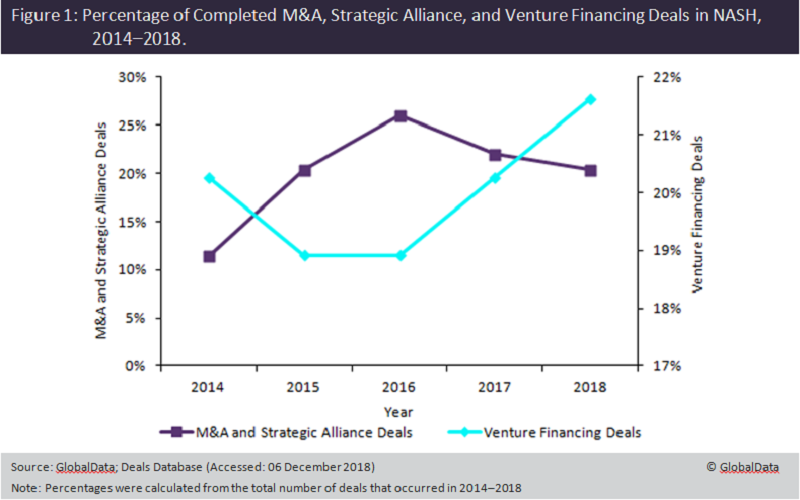
Figure 1 shows mergers and acquisitions (M&A) and strategic alliance deal activity for NASH, as well as venture financing activity. Both the attractive market potential and the significant unmet needs in this space, which has no FDA-approved products, stimulated M&A and strategic alliance deal activity between 2014 and 2016. In this time, several big biopharma and biotechs, such as Gilead Sciences, Novartis, and Allergan, entered this space in an attempt to gain a first-mover advantage.
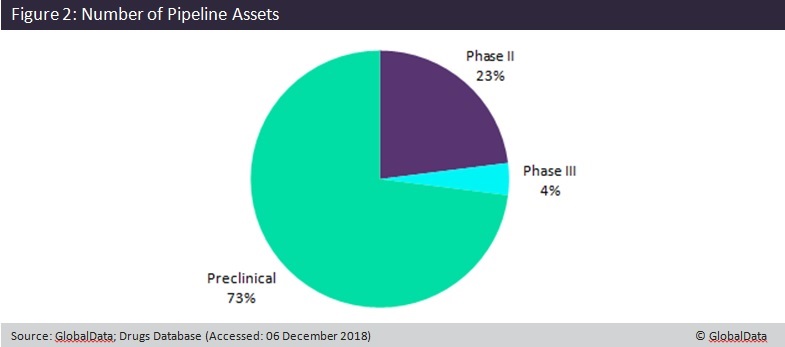
However, M&A and strategic alliance deal activity began to decrease after 2016. One potential explanation for this is the high proportion of drugs in preclinical stages of development due to the complexity of the disease. Figure 2 shows the 127 drugs currently in preclinical trials, compared to just 7 in Phase III. This is reflected by a simultaneous increase in early-stage venture financing activity since 2016, suggesting the industry is still in a pioneering phase of growth.
Furthermore, due to the complexity of the disease, key players in the market are seeking to develop combination therapies to target multiple stages of NASH progression to produce a successful treatment. For example, on October 29, Pfizer and Novartis collaborated to develop a therapy combining their solid NASH pipelines, including Novartis’ emricasan. As the industry matures and companies are able to capitalise on the data received from clinical trials, the levels of later-stage partnering activity are likely to rise.
Register as Visitor to CPhI China 2019!
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


