-
Nicox broadens two agreements with Ocumension in South East Asia

March 12, 2020France-headquartered Nicox has expanded two licensing agreements with Chinese Ocumension Therapeutics for two ophthalmology drugs. -
Nicox signs agreement for ZERVIATE in South Korea
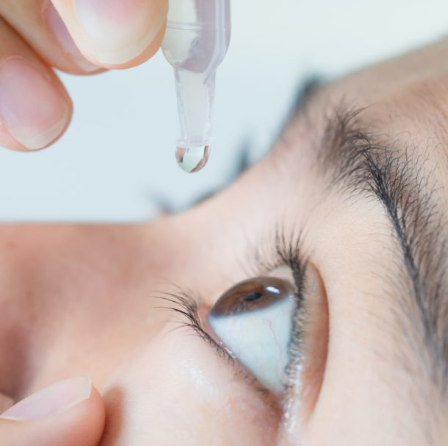
December 18, 2019Samil Pharmaceutical will receive exclusive rights to develop and commercialize ZERVIATE in South Korea, where the market for allergic conjunctivitis was worth nearly €31 million for the 12 months to Q3 2019. -
FDA Approves Zerviate
June 08, 2017Alkermes plc (NASDAQ: ALKS) today announced that the U.S. Food and Drug Administration (FDA) has approved two-month Aristada (aripiprazole lauroxil) extended-release injectable suspension for the treatment of schizophrenia. -
FDA Approves Zerviate
June 07, 2017Nicox Receives FDA Approval of Zerviate (cetirizine ophthalmic solution) 0.24%
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








