-
NICE backs Lilly’s Taltz for axial spondyloarthritis
pharmatimes
June 18, 2021
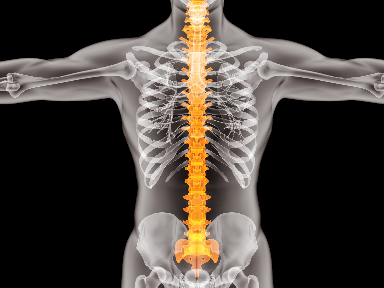
The National Institute for Health and Care Excellence (NICE) has recommended Eli Lilly’s Taltz for the treatment of active ankylosing spondylitis (axSpA).
-
Lilly bags expanded EU approval for Taltz
pharmatimes
June 08, 2020
The European Medicines Agency has expanded the reach of Lilly's Taltz (ixekizumab) to include the treatment of adults with active ankylosing spondylitis and active non-radiographic axial spondyloarthritis (nr-axSpA).
-
FDA approves Lilly’s plaque psoriasis drug Taltz for children
pharmaceutical-technology
April 01, 2020
The US Food and Drug Administration (FDA) has approved Eli Lilly’s Taltz (ixekizumab) to treat moderate to severe plaque psoriasis in children aged six to below 18 years.
-
Positive results for Eli Lilly’s Taltz in late stage non-radiographic axial spondyloarthritis trial
pharmatimes
April 24, 2019
Eli Lilly has announced that its humanised monoclonal antibody, Taltz (ixekizumab), yielded good results in a Phase III trial.
-
Lilly’s Taltz meets endpoints in Phase III study for nr-axSpA
pharmaceutical-technology
April 24, 2019
Global pharmaceutical company Eli Lilly has announced top line results for Taltz (ixekizumab) in non-radiographic axial spondyloarthritis (nr-axSpA).
-
Novartis’ Cosentyx lands first-in-class Chinese psoriasis nod
fiercepharma
April 10, 2019
Novartis’ psoriasis blockbuster Cosentyx has beaten out rivals from Eli Lilly and Johnson & Johnson as the first interleukin-17A inhibitor approved in China.
-
Eli Lilly's Taltz pads case for a shot at Novartis' Cosentyx in ankylosing spondylitis
fiercepharma
June 29, 2018
Eli Lilly’s Taltz is already up against Novartis’ immunology blockbuster Cosentyx in psoriasis and psoriatic arthritis. Now, Lilly looks poised to challenge the Swiss drugmaker in a third indication, too.
-
Taltz Receives FDA Approval for Active Psoriatic Arthritis
americanpharmaceuticalreview
December 06, 2017
Eli Lilly and Company announced the U.S. Food and Drug Administration (FDA) has approved Taltz (ixekizumab) injection 80 mg/mL for the treatment of adults with active psoriatic arthritis (PsA).





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








