-
FDA Grants Rare Pediatric Disease Designation to Elamipretide for Barth Syndrome

March 05, 2020Stealth BioTherapeutics announced the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease (RPD) designation for elamipretide for the treatment of Barth syndrome. -
Renal Anomaly on Fetal Scan May Up Risk for Admission for Child
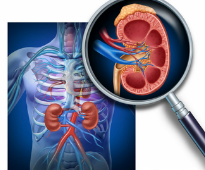
August 16, 2019Renal pelvis dilatation (RPD) at the fetal anomaly scan (FAS) and persistent dilatation in later pregnancy or postpartum is associated with an increased risk for hospital admission in early childhood...
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








