-
Prestige Biopharma drug gets positive EMA opinion on Orphan Designation to treat pancreatic cancer
expresspharma
October 19, 2020
Prestige BioPharma announced that the European Medicines Agency (EMA) Orphan Drug Commission (COMP) has granted a positive opinion for an Orphan Drug Designation (ODD) status to its first-in-class anti-PAUF monoclonal antibody, PBP1510, for the ...
-
Prestige Biopharma and Pharmapark Announce License and Supply Agreement to Commercialize Prestige's Trastuzumab Biosimilar in the Russian Federation
b3cnewswire
July 08, 2019
Prestige BioPharma and Pharmapark LLC today announced that the two companies have entered into a binding agreement for the exclusive partnership and supply for the commercialization of Prestige BioPharma´s Trastuzumab biosimilar in the Russian Federation.
-
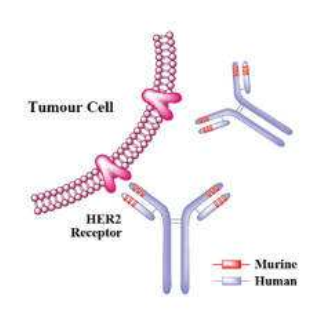
Prestige BioPharma Reports Positive Top-Line Phase III Results for Tuznue® (Trastuzumab Biosimilar)
b3cnewswire
June 24, 2019
Prestige BioPharma announced positive top-line results from a Phase III global clinical trial (Troika) evaluating the efficacy, safety, and pharmacokinetics (PK) of biosimilar candidate HD201 to Herceptin (trastuzumab).
-
EMA accepts Prestige Biopharma's MAA for review
biospectrumasia
May 29, 2019
HD201 is Prestige’s first biosimilar to receive a positive Committee for Medicinal Products for Human Use (CHMP) opinion for marketing authorization from the EMA.
-
Prestige BioPharma Partners with Cipla Ltd to Market Key Cancer Biosimilar
b3cnewswire
December 17, 2018
Prestige BioPharma (“Prestige”) announced today that it has reached a licensing agreement with Cipla Limited (“Cipla”) for its trastuzumab biosimilar (HD201) under which......
-
Prestige Biopharma and Alvogen Announce License and Supply Agreement to Commercialize Prestige´s Trastuzumab Biosimilar (Hervelous™) in Central and Eastern Europe
b3cnewswire
July 04, 2018
Prestige BioPharma and Alvogen today announced that the two companies have entered into a binding agreement for the exclusive partnership and supply for the commercialization of Prestige BioPharma´s Trastuzumab biosimilar (HD201; Hervelous™) in Central an
-
Prestige BioPharma, Alvogen Enter Agreement
contractpharma
July 03, 2018
Prestige BioPharma and Alvogen have entered into a binding agreement for the exclusive partnership and supply for the commercialization of Prestige BioPharma´s Trastuzumab biosimilar (HD201; Hervelous™) in Central and Eastern Europe.

 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








