-
PharmaCyte Biotech Announces Closing of $70 Million Registered Direct Offering Priced At-the-Market under Nasdaq Rules

August 24, 2021PharmaCyte Biotech, Inc. (NASDAQ: PMCB) today announced the closing of its previously announced registered direct offering priced at-the-marked under Nasdaq rules, of 14,000,000 shares of the Company's common stock -
PharmaCyte Biotech submits IND to FDA for clinical trial in inoperable pancreatic cancer
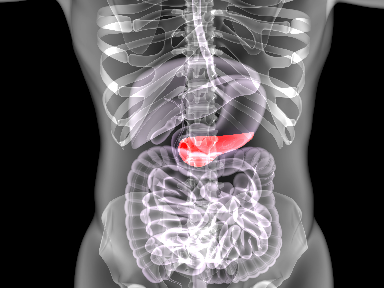
September 08, 2020PharmaCyte Biotech has submitted an Investigational New Drug application (IND) to the U.S. Food and Drug Administration (FDA) for a planned Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC). -
PharmaCyte Biotech Successfully Completes Multiple Course Ifosfamide Study
April 18, 2018PharmaCyte Biotech, Inc.today announced that it has successfully completed a “multiple course ifosfamide study” required by the U.S. Food and Drug Administration (FDA).
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








