-
Mycenax becomes Taiwan, China's first CDMO company to complete the CHO-S and CHOZN GS Cell Line Development with the Beacon(R) Optofluidic System
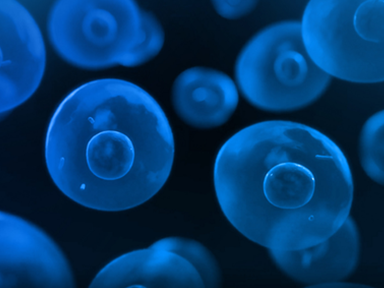
May 18, 2021Mycenax becomes one of the first CDMO companies in Asia to adapt the Beacon Optofluidic system to cell line development services. -
Mycenax’s Actemra biosimilar edges closer to approval

January 02, 2019Mycenax’s biosimilar of Roche’s Actemra (tocilizumab), LusiNEX, has been shown to match its originator’s profile in a phase I PK trial, meaning it is on track to become the first approved biosimilar for the drug.
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








