-
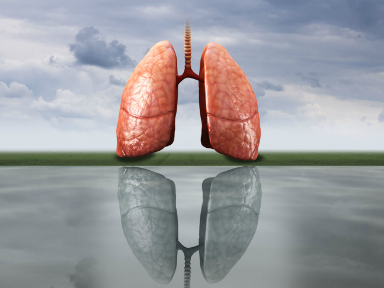
FDA Expands Collaboration with CN Bio to Investigate Lung-On-A-Chip Model for Inhaled Drug Evaluation Applications
americanpharmaceuticalreview
May 07, 2021
CN Bio, a developer of single- and multi-organ-on-chip microphysiological systems (MPS) that improve the accuracy and efficiency of drug discovery, announced that the U.S. Food and Drug Administration (FDA) has extended their research collaboration ...
-
Vertex told to reveal CF drug price, as MPs begin inquiry
pharmaphorum
December 04, 2018
UK politicians could go public with the price of Vertex’s cystic fibrosis drug Orkambi, if the company does not strike a deal giving NHS patients access by the end of the month.
-
MPs voice concern over clinical trials transparency
pharmatimes
November 05, 2018
MPs have expressed concern that nearly half of clinical trials in the UK fail to publish their results, “presenting risks to human health and increasing...
-
MPs vote for UK to remain part of EMA
pharmatimes
July 19, 2018
MPs have voted 305-301 that the UK should negotiate to remain part of the European Medicines Agency following Brexit.
-
Ministers write letter to Vertex over Orkambi debate
pharmatimespharmatimes
July 16, 2018
Ministers from the Department of Health have written a letter asking that Vertex bring negotiations with NHS England over access to cystic fibrosis drug Orkambi “to an urgent resolution”.
-
MPs keen for close collaboration with EMA post Brexit
pharmatimes
July 04, 2017
British MPs say they are keen to secure a close working relationship with the European Union on drug regulation post Brexit to protect patient health and investment in the UK’s life sciences sector.
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








