-
Everest Medicines Receives Orphan Drug Designation from the Ministry of Food and Drug Safety in South Korea for Sacituzumab Govitecan-Hziy in Metastatic Triple-Negative Breast Cancer

May 06, 2021Everest Medicines, a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products in Greater China and other parts of Asia, announced today that the Ministry of Food and Drug Safety (MFDS) in South Korea ... -
Celltrion receives Korean MFDS approval to initiate trial of Covid-19 antiviral antibody treatment candidate
September 02, 2020Celltrion Group announced that the Korean Ministry of Food and Drug Safety (MFDS) has approved the company’s Investigational New Drug (IND) application for a Phase I clinical trial of CT-P59, a COVID-19 antiviral antibody treatment candidate. -
VUNO Med(R)-Fundus AI(TM) receives MFDS Regulatory Approval as Class III Medical Device
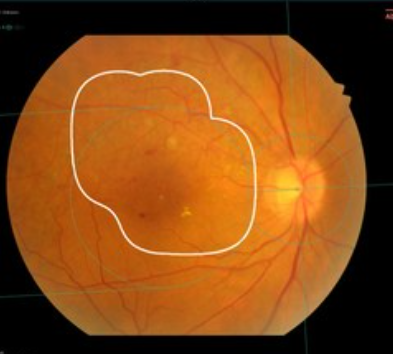
April 14, 2020VUNO, a member company of the Born2Global Centre, announced that their AI based screening solution for the fundus, VUNO Med®-Fundus AI™ is approved as a Class III medical device by the Ministry of Food and Drug Safety. VUNO Med®-Fundus AI™ is the first ev
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








