-
Elekta radiotherapy system designed for changing cancer landscape now available for U.S. patients

June 17, 2021Elekta (EKTA-B.ST) announced today that its Elekta Harmony* radiation therapy system recently received U.S. FDA 510(k) clearance, paving the way for U.S. clinics to harness the system to treat a comprehensive range of indications using the latest ... -
Acadia Pharmaceuticals Receives Complete Response Letter from U.S. FDA for Supplemental New Drug Application for Pimavanserin for the Treatment of Hallucinations and Delusions Associated with Dementia

April 09, 2021Acadia Pharmaceuticals Inc. announced that the Company has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding its supplemental New Drug Application (sNDA) for NUPLAZID® (pimavanserin) for the treatment ... -
Akero Initiates Phase 2b HARMONY Study of Efruxifermin for NASH
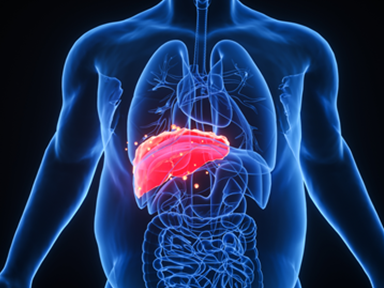
March 01, 2021Akero Therapeutics has screened the first patients for its Phase 2b HARMONY study evaluating efruxifermin (EFX), the company's lead product candidate for the treatment of NASH.
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








