-
Ortho's Total Antibody Test for COVID-19 Receives Emergency Use Authorization from FDA

April 15, 2020The U.S. Food and Drug Administration (FDA) today announced it granted Emergency Use Authorization to Ortho Clinical Diagnostics' total antibody assay for COVID-19—the VITROS® Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack and Calibrators. -
Synapse Biomedical Receives FDA Emergency Use Authorization for New System to Assist in Weaning Patients off of Ventilators During COVID-19 Pandemic
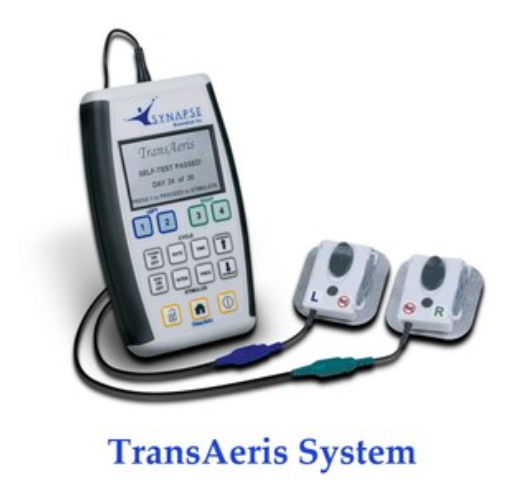
April 15, 2020Synapse Biomedical, Inc. has received FDA Emergency Use Authorization for the emergency use of its TransAeris® DPS, to assist in weaning patients determined by their healthcare provider to be at high risk of weaning failure off of ventilators in healthcar
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025








