-
DiscGenics Completes Patient Enrollment in Japanese Clinical Study of Cell Therapy for Disc Degeneration as US Study Achieves One-Year Follow-up Milestone
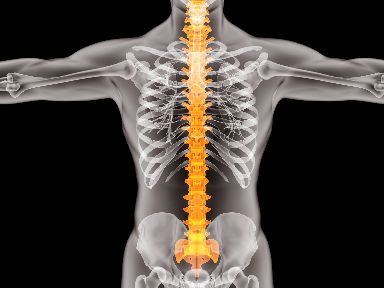
April 27, 2021DiscGenics, Inc. today announced that it has completed enrollment in its Japanese safety study of IDCT, an allogeneic, injectable Discogenic Cell therapy for lumbar disc degeneration, a major cause of chronic low back pain. -
DiscGenics treates First Patients in Japanese Clinical Trial of Cell Therapy for DDD

August 08, 2019The prospective, randomized, double-blinded, sham-controlled study is designed to evaluate the safety and preliminary efficacy of IDCT at two dosage levels in subjects with single-level, symptomatic lumbar DDD, a major cause of chronic low back pain -
DiscGenics Announces First Patient Treated in U.S. Trial of IDCT for Degenerative Disc Disease
April 27, 2018DiscGenics announced the first patient has been treated in its phase I/II U.S. clinical trial of IDCT for mild-to-moderate degenerative disc disease (DDD).
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








