-
CNMPA approves Agilent's PD-L1 Companion Diagnostic
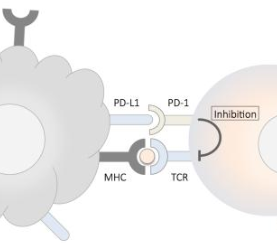
October 08, 2019The assay is now approved as a companion diagnostic to identify patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) whose tumors express PD-L1 (tumor proportion score [TPS] >1%) for first-line treatment with single-agent KEYTRU
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








