-
Research and Application of Artemisia Argyi
Xiaonisha
August 12, 2024
Mugwort, also known as fragrant mugwort or Artemisia argyi, is a herbaceous plant belonging to the Compositae family.
-
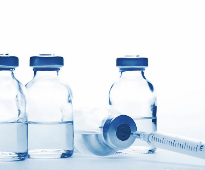
How to Select Blister Packaging machine for your Application
Muhammad Asim Niazi
July 03, 2024
Blister packaging machine is an integral equipment in the pharmaceutical industry, and it is used to pack the oral form of pharma drug products such as tablets into suitable packaging material.
-
South Africa Ups Registration of New Pharmaceutical Products
SHEM OIRERE
March 13, 2023
SAHPRA has recently announced completion of a review of 16,000 applications for new products and variations nearly four years after it took over from the former regulator, the Medicines Control Council.
-
Call for Application for 2020 Tsinghua Amgen Scholars Program
prnasia
January 15, 2020
15 excellent candidates will be selected worldwide for the coming program in 2020, whose roundtrip tickets to Beijing, part of meal allowance, student apartment or on-campus hotel are covered, and stipends will be offered.
-
DEA to Approve Dozens More Growers for Marijuana Research
drugs
August 28, 2019
The number of marijuana growers allowed to produce the drug for U.S. government-approved research will be expanded from one to 34, officials say.
-
FDA and EMA align on 90 percent of marketing authorisation decisions
europeanpharmaceuticalreview
August 23, 2019
A study has found that between 2014 and 2016, the EMA and FDA had very little divergence in their marketing authorisation decisions.
-
Submission and acceptance of application for the production of new drug for Yisaipu(R)aqueous injection solution
prnasia
August 06, 2019
3SBio Inc. (01530.HK), a leading biopharmaceutical company in the PRC, announces today that an application for the production of new drug has been submitted to the National Medical Products Administration for ...
-
BioInvent Receives TAK-169 IND Application Milestone Payment
americanpharmaceuticalreview
July 08, 2019
BioInvent announced it will receive a $0.5 million milestone payment related to the acceptance by the U.S.
-
Steriline products and solutions: Robotic applications
europeanpharmaceuticalreview
May 22, 2019
Steriline products and solutions: Robotic applications.
-
Celgene Corporation and Acceleron Pharma Announce Submission of Luspatercept Biologics License Application to U.S. FDA
drugs
April 11, 2019
Celgene Corporation and Acceleron Pharma Announce Submission of Luspatercept Biologics License Application to U.S. FDA.









 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








