-
Janssen Submits Application to European Medicines Agency Seeking Approval of STELARA (ustekinumab) for Treatment of Adult Patients with Moderately to Severely Active Ulcerative Colitis
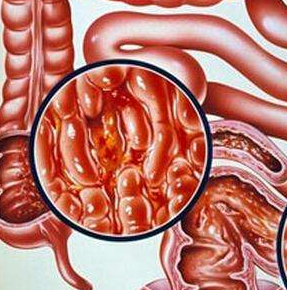
January 09, 2019The Janssen Pharmaceutical Companies of Johnson & Johnson announced today the submission of a Group Type II Variation Application to the European Medicines Agency (EMA) seeking approval of STELARA®(ustekinumab) for the treatment of adults with moderately -
Janssen Submits Application to European Medicines Agency Seeking Approval of STELARA (ustekinumab) for Treatment of Adult Patients with Moderately to Severely Active Ulcerative Colitis

January 09, 2019The Janssen Pharmaceutical Companies of Johnson & Johnson announced today the submission of a Group Type II Variation Application to the European Medicines Agency (EMA) seeking approval of STELARA®(ustekinumab) for the treatment of adults with moderately -
FDA accepts sNDA for Xeljanz for the treatment of adult patients with active ulcerative colitis
July 17, 2017Anticipated Prescription Drug User Fee Act (PDUFA) action date for the sNDA is March 2018 .
 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








