
Deepak HegdeDecember 13, 2023
Tag: spray drying , Solubility , bioavailability
Spray drying, an innovation in the development and manufacturing field has been gaining importance over the last few years, as an increasing number of drug products have been getting approved by regulators over the world and reaching the market. This series of two article on this topic reviews this important topic.
The first article provides an outline of the issue of poor solubility and bioavailability, how spray drying has been addressing this critical issue, typical spray drying process and application of spray drying from an early to late phase of product development.
The second article in the series on spray drying would address some of the practical considerations in application of spray drying to later phases of development, proprietary platforms in the industry, some products which have been commercialized for small molecules as well as application of spray drying to protein products, challenges and some products which have been commercialized for proteins.
Around 50% of drugs on the market and nearly 90% of molecules in the discovery pipeline are estimated to be poorly water soluble. An increasing number of new chemical entities (NCEs) with poor physical, chemical, and biopharmaceutical properties are contributing to high failure rates due to efficacy issues. A key factor has been poor solubility leading to low bioavailability, resulting in suboptimal drug delivery, ineffective drug efficacy, and side effects.
The Biopharmaceutical Classification System (BCS) classifies poorly soluble compounds as Class II (poor solubility and high permeability) and Class IV drugs (poor solubility and poor permeability). Drug substances are considered highly soluble when the largest dose of a compound is soluble in <250ml of water over a pH range of 1-7.5. Highly permeable compounds are classified as those compounds that demonstrate over 90 per cent absorption of the administered dose. Compounds with solubilities below 0.1mg/mL face significant solubilization obstacles, and sometimes even compounds with solubilities below 10mg/mL present difficulties related to solubilization during formulation. Figure 1 summarizes various approaches used to shift the solubility characteristics of a poorly soluble drug.
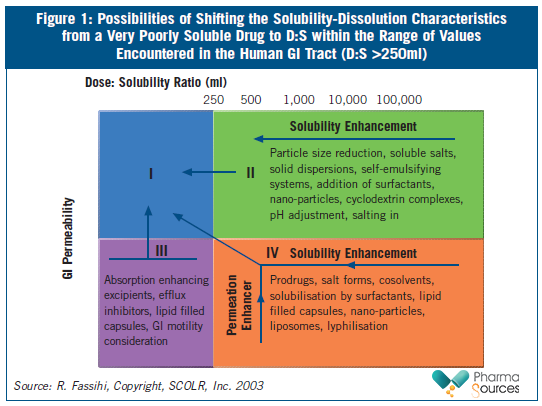
In 2010, the Developability Classification System (DCS) was proposed by J. Dressman1. The DCS was designed to close the gap between the biopharmaceutics classification system, which is aimed at guiding regulatory decisions about well-characterized drugs, and the need for early evaluation of drug candidates with respect to their suitability for oral delivery. In this classification, drugs are partitioned into four classes based on their jejunal permeability and the volume of simulated intestinal fluid needed to dissolve an entire single dose.
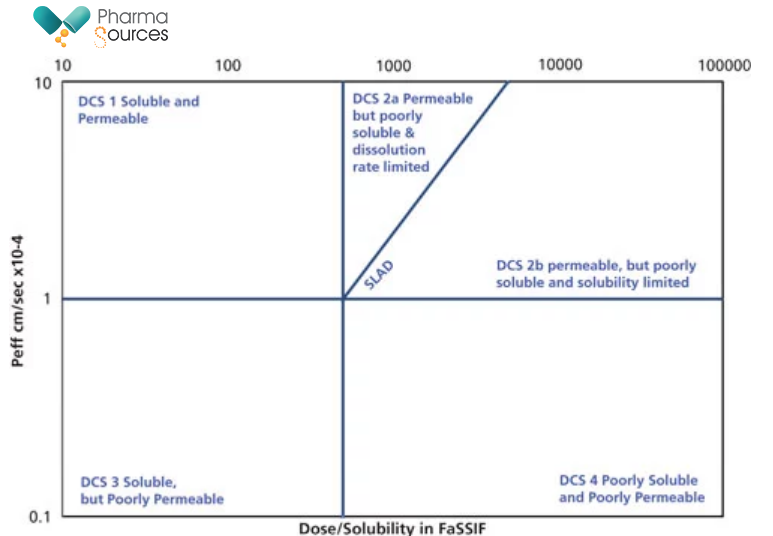
Fig 2: Developability Classification System (DCS) classification chart.
(Source: Pharmaceutical Technology-04-02-2018, Volume 42, Issue 4, Pages: 72–77.)
DCS calculates the solubility limited absorbable dose (SLAD), above which no more drug is expected to dissolve during the transit time through the small intestine, and this is used to split DCS Class II into molecules with dissolution rate limitations (DCS Class IIa) and solubility limitations (DCS Class IIb) to oral absorption. Here, it is important to remember that dose means the maximum dose taken by a patient at one time (not the highest strength dosage unit), and this number is increased during ascending dose studies.
A classification of GlaxoSmithKline candidates-and likely to be representative of the industry at large-found that 65% of NCEs could be classified as DCS IIa/IIb (dissolution rate or solubility limited)2. With my experience working with molecules at GSK, it was observed that for the same molecule the lower dose could be a well-soluble DCS Class I while the higher dose could be DCS IIb. For a compound classified as DCS IIb, employing particle size reduction alone or dosing as a solution (e.g., in polyethylene glycol) would not be expected to increase solubility, and solubility-enhancing technologies such as amorphous dispersion, lipid formulation, or perhaps co-micronization should be considered instead, as these technologies are likely to have a far greater impact on the SLAD.3
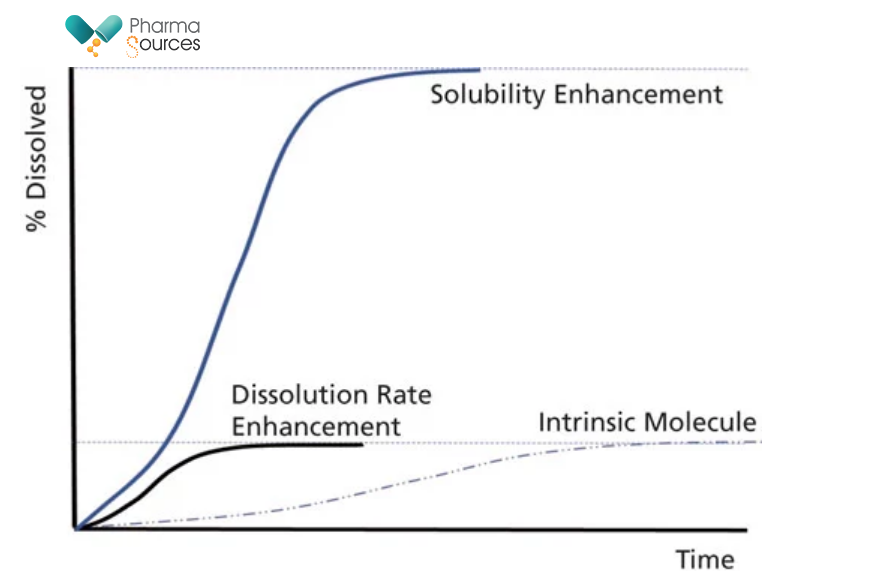
Fig 3: Schematic of the difference between faster dissolution rate and solubility enhancement
(Source: Pharmaceutical Technology-04-02-2018, Volume 42, Issue 4, Pages: 72–77.)
Use of amorphous forms has emerged as a preferred option for addressing the issue of poor solubility. Amorphous of drug molecules are high energy forms. Since there is no crystal lattice structure (no long-range order), the energy barrier to dissolution is much reduced. However, amorphous forms are inherently unstable, and will tend to revert to the most stable (least soluble form). Hence, amorphous forms most often need to be stabilized as solid solutions or polymer dispersions (Amorphous Solid Dispersions-ASD’s). Typically, amorphous forms can give up to a 10–15 fold increase in bioavailability. Classical methods of manufacture of amorphous forms include spray drying and Hot-Melt Extrusion.
For early phase development of NCE’s, spray drying has emerged as a method of choice for manufacture of amorphous forms. A major reason for this is the low amounts of API required for formulation development using spray drying approach. For NCE’s, at an early stage where compounds are often synthesized by the medicinal chemistry synthetic route and limited availability of compounds is often a constraint. Another key reason is the relatively quick speed of development of ASD’s using spray drying approach. A final reason is that it is relatively quicker for scale-up for later phases. While spray drying does require expertise and experience for handling, especially for later phases, it is still a preferred option.
Typical Spray drying process.
Most typical spray drying processes utilize polymers which are dissolved into one or often into a combination of more than one organic solvents. The polymer helps to stabilize the Active Pharmaceutical Ingredient (API’s) amorphous state by physically separating the drug’s molecules and due to the random order of the API molecules inside the polymer matrix, the ASDs completely release drugs without inducing re-crystallization. The API and polymer solution is then pumped into a drying chamber as a fine mist. The hot processed gas enters through the top of the chamber (red arrow) and vaporizes the solvent to produce an ASD.
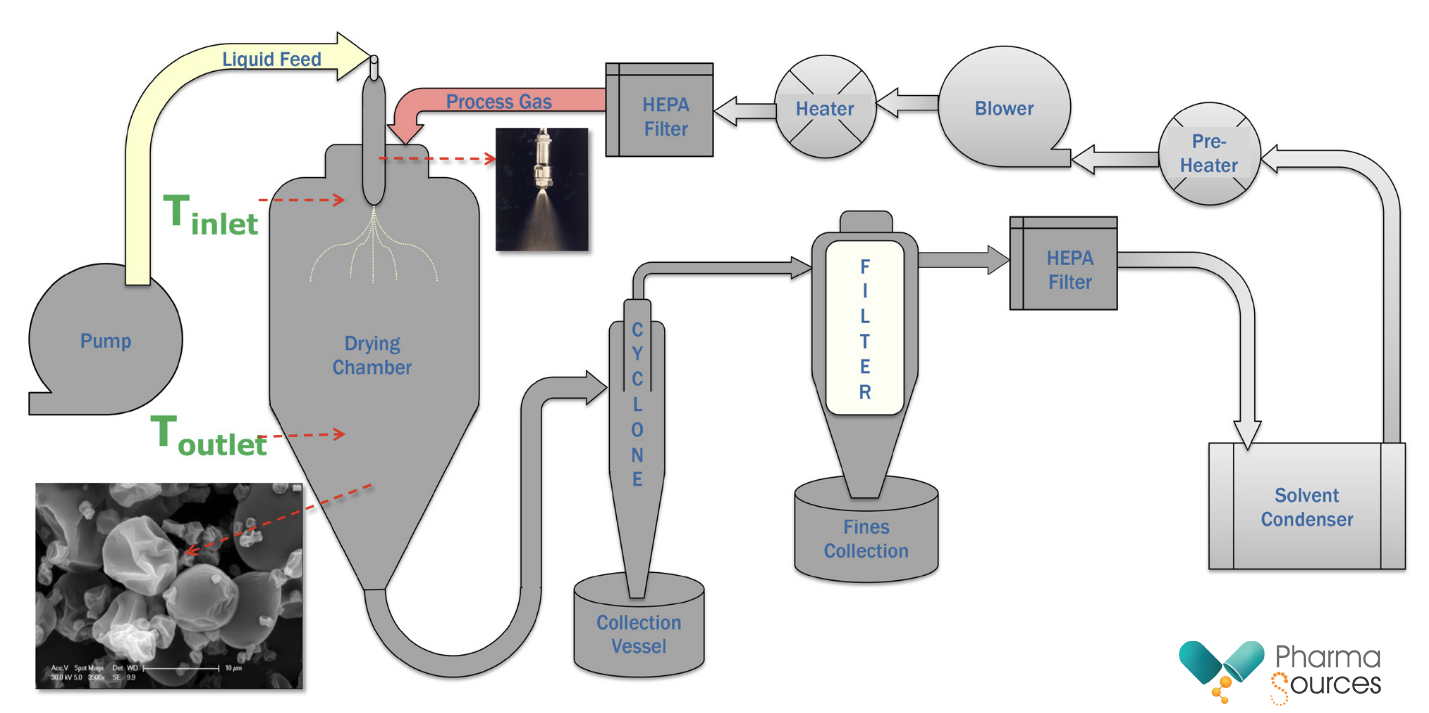
Fig 4: Schematic of a typical spray drying process
(Source: eBook-Pharmaceutical Technology-May 2020, Page:3)
The ASD particles, which are heavier than the air, spiral down the sides of the cyclone into the collection vessel. If the particles are correctly formed and large enough, very little material gets collected in the secondary collection vessel. The processed gas stream then rises through the middle of the cyclone and through a cartridge filter where fines are collected. The condenser removes and collects most of the vaporized solvent from the processed gas. After recycling through a fan box and heat exchanger, the processed gas is then returned to the chamber. The entire system operates as a closed loop and can operate as a continuous process. The ASD often contains solvents as residuals and a secondary drying process may be necessary to reduce the residual solvent levels to acceptable levels.
Application from early to late phase development
At a very early phase, where extremely low amounts of API may be available and to explore possible increase in solubility for solubility using ASD’s, use of Miniaturized Solvent Casting (MSC)4 or film casting is often used in the industry. This acts as a rapid screening technique for drug–polymer amorphous dispersions made by solvent removal techniques. Dissolution studies are performed on the films to check for increase in solubility and accelerated stability of ASD films are performed to check for stability of ASD films. The method is suited to developability assessment as a risk-reduction tool during the formulation of drug product. This technique is rapid, repeatable and presents a very practical approach. Once confirmed by this technique, the next step is to manufacture ASD’s using spray drying.
Typically, commonly used solvent systems are screened to identify solvents that will improve the API’s solubility and stability. Based on solvent screening, a single solvent, or binary or tertiary mixture of solvents such as acetone, DCM or methanol could be used. Once solvent screening is completed, based on the physicochemical properties of the API and the selected solvent, polymer selection is the next step. At this stage, several factors such as stability of the API during storage in the solution to be spray dried, over a period of few days, as well as solid loading possible in the spray drying solution are often evaluated to ensure that the spray drying process is feasible and will be efficient. At this stage it is common to use spray driers like ProCepT 4M8-TriX™ for making very small amounts of ASD’s for evaluation and then scale up using Buchi B-290™ lab spray driers. Typically, these are used for making non-GMP batches used for Pharmacokinetic (PK) studies in animals to check for increase in bioavailability using ASD Approach.
A limitation with laboratory scale spray driers is that they cannot be run continuously to generate larger scale material for development batches and hence these is a need to scale up with other models of spray driers. Spray driers like GEA SDMICRO™ can run continuously in a closed loop system and manufacture large batches and smaller GMP batches which can be used for Phase I studies. Going past Phase I it is common to use spray driers like GEA NiroMOBILEMINOR™. In comparison to laboratory-scale spray drying, its use at pilot scale or industrial scale is more challenging. More attention should be paid to issues like mixing and homogeneity of the feed, pumping, atomization, contamination, etc. Another factor worth noting is that industrial-scale spray dryers are usually suitably equipped with devices to enable the continuous collection and post manufacturing drying the ASD’s. These drying devices may provide vast improvement of stability in ASD’s, which is generally neglected in lab-scale studies. Moving on to commercial scale, GEA Niro has the range of spray driers ranging from PHARMA-SD® type PSD-1 to PHARMA-SD® type PSD-6 to address different commercial needs. While the author mentions about certain GEA Niro products in this article, these are purely based on the author’s personal experience with these products. There are other manufacturers who address spray drying manufacturing needs at different scales.
Continue to read:
1. J.M. Butler and J.B. Dressman, J Pharm Sci. 99, 4940-54 (2010).
2. K. Smietana, et al., Nat Rev Drug Discov. 14, 455-6 (2015)
3. Stephen Tindal, Ronak Savla, Pharm. Technol, Volume 42, Issue 4, 72(2018)
4. M.Davis et al, Chemical Engineering Research and Design,110, Pages 192-199 (2016)

Deepak Hegde, Ph.D., M.F.M, is an industrial pharmacist by training. He has a been involved in development and commercialization of both innovative and generic drugs from a very early phase of development to technical transfers for commercial manufacturing sites, for the past 25 years. During his career, he has worked at Rhone Poulenc, Novartis (Sandoz), USV Ltd., WuXi AppTec, GSK & EOC Pharma. He is currently working with Shenzhen Pharmacin Co. Ltd. as Senior Vice President-Technology & Manufacturing.
Email: deepak.hegde@hlkpharma.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


