Suzanne ElvidgeFebruary 21, 2024
Tag: Nanoparticles , Drug Delivery
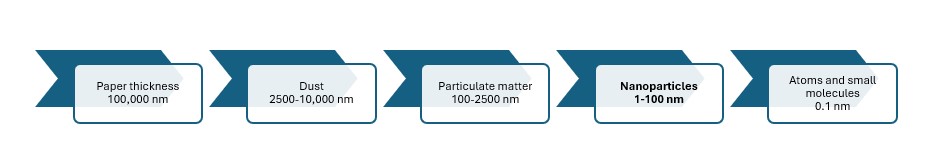
Nanoparticles are small particles between 1 nm and 100 nm, and there is growing interest in nanotechnology in energy, electronics, materials science and biomedicine.
Source: TWI
The global healthcare nanotechnology market was worth around $320 billion in 2023, and could be worth as much as $894 billion by 2032. Nanoparticles have potential in imaging, diagnostics, bio-implants and tissue engineering. One of their growing roles is in targeted and controlled drug delivery.
The small size of a nanoparticle means that they can evade scavenging by the mononuclear phagocyte system in the liver and filtration by inter-endothelial cells in the spleen, increasing the systemic circulation time. This increases the amount of drug available for absorption.
The large surface area to volume ratio of a nanoparticle means that they have a large loading capacity for their size.
Nanoparticles can be engineered to deliver drugs to specific cells or tissues. By taking therapeutics, particularly drugs with toxic effects and narrow therapeutic windows, directly to their site of action, allows doses to be higher where needed and lower in the systemic circulation. This can increase efficacy and reduce side effects.
Targeted delivery can be achieved by attaching targeting molecules to the surface of the nanoparticle, such as antibodies, peptides, lectins, saccharides or hormones. By adding polymers that are sensitive to pH or temperature, nanoparticles can release their contents at specific locations in the body. Targeted delivery can also be passive; nanoparticles can accumulate in places with vascular abnormalities, such as tumours. Tumour vessels can also be permeable and the lymphatic drainage is lower than in healthy tissues. This is known as the enhanced permeability and retention effect (EPR), and results in improved drug delivery to tumours.
Nanoparticles can also be engineered to deliver drugs gradually over a period of time, reducing the frequency of dosing, improving convenience for patients and carers/healthcare professionals, and potentially leading to better drug adherence.
The types of nanoparticles in development or on the market in healthcare applications include:
● nanocrystals
● liposomes
● polymeric micelles/nanoparticles
● nanoemulsions and solid lipid nanoparticles
● dendrimers
● metal nanoparticles
● carbon nanotubes
● quantum dots.
Reducing drug particle size to below 100 nm can change the solubility and dissolution of poorly soluble drugs, providing potential for oral, injectable and topical administration.
Examples of nanocrystals include:
● Invega Hafyera (Janssen, launched in 2021) – paliperidone palmitate nanocrystals for the intramuscular treatment of schizophrenia
● Apretude (Viiv Healthcare, launched in 2021) – cabotegravir nanocrystals for the gluteal intramuscular treatment of HIV-1 infection
Liposomes are spheres made up of a lipid bilayer. They self-assemble from natural or synthetic phospholipids, and can carry drug molecules within the lipophilic bilayer or the aqueous core. Liposomes can be administered via ocular, oral, pulmonary, transdermal and parenteral routes. Changing the formulation of the liposome can prolong its circulation, change the biodistribution profile, increase drug effectiveness and allow targeting to specific tissues. Liposomes have poor stability during storage, but this can be improved by freeze-drying.
Examples of liposomal nanoparticles:
● Vyxeos (Jazz Pharmaceuticals, launched in 2017) – daunorubicin/cytarabine liposome for the intramuscular, intrathecal or subcutaneous treatment of acute myeloid leukaemia (AML)
● Comirnaty (Pfizer and BioNTech, launched in 2021) – intramuscular COVID vaccine
A polymeric micelle is formed from amphiphilic polymers. These self-aggregate into spheres, with the hydrophilic part of the molecule in the core and the hydrophilic potion on the surface or shell. One or more drugs with poor solubility sit in a depot in the core, and the shell helps the nanoparticle stay in the blood circulation for longer by limiting opsonin absorption. Polymeric micelles are also used in oral drug delivery, by stabilising the drug in the gastrointestinal tract.
Examples of polymeric micelles/nanoparticles:
● Zilretta (Flexion Therapeutics, launched in 2017) – triamcinolone acetonide polymeric nanoparticles as an extended-release intra-articular injection for osteoarthritis knee pain
● Plegridy (Biogen, launched in 2014) – pegylated IFN-β-1a polymeric nanoparticles as subcutaneous treatment for multiple sclerosis
● Abraxane (Celgene, launched in 2005) – paclitaxel micellar nanoparticles as an intravenous treatment for breast, non-small cell lung, pancreatic and ovarian cancer
Nanoemulsions are stable mixtures of nanoparticle-sized droplets of hydrophobic liquids dispersed in a hydrophilic liquid (oil-in-water) or hydrophilic droplets in a hydrophobic liquid (water-in-oil). Solid lipid nanoparticles are similar to oil-in-water nanoemulsions, but replace the liquid lipid with a solid form. The lipid phase of the emulsion can be used to deliver a hydrophobic drug. Nanoemulsions have potential for topical, intravenous, ocular, nasal and oral delivery.
Example of nanoemulsions:
● Estrasorb (Novavax, launched in 2003) – oestradiol nanoemulsion as a topical oestrogen therapy
Dendrimers are spherical, symmetrical, branched nanoparticles that can incorporate drugs. By improving the pharmacokinetics and pharmacodynamics of the drug, they can improve its bioavailability and stability. They can also control drug release and target drug delivery. They have potential for oral, nasal, ocular and transdermal drug delivery.
Example of dendrimers:
● VivaGel (Starpharma, launched in 2006) – SPL7013 as an intravaginal topical prevention against HIV and HSV infection.
Metal nanoparticles, which include particles of gold, silver, iron or iron oxide, zinc, titanium, cerium oxide, nickel, copper, magnesium, barium, calcium, and bismuth, can carry drugs and targeting molecules, and can act as therapeutics in their own right (for example iron in anaemia). Changing the coating of metal nanoparticles can alter their pharmacokinetics, for example reducing uptake by the mononuclear phagocyte system.
Metal nanoparticles include magnetic nanoparticles, most commonly based on iron, nickel or cobalt. These can target specific locations when guided using a magnet. The particles can carry drugs, or can raise the temperature locally to kill cells using magnetic hyperthermia.
Examples of metal nanoparticles:
● Monofer (Pharmacosmos, launched in 2013) – iron nanoparticles for the treatment of iron deficiency anaemia
Carbon nanotubes have a high surface area to volume ratio, which means that they have potential for a high drug or gene cargo. They are also strong, conductive and biocompatible, and can release their payload in response to triggers including pH, redox potential changes, enzymatic activation, thermal gradients, magnetic fields, light and ultrasound. However, as they are not biodegradable, there are environmental and toxicological concerns.
Quantum dots are nanoscale semiconductor crystals that fluoresce brightly and intensely. They have potential as theranostics, where they can act as both the drug carrier and the fluorescent label. They also have potential as sensors to detect biomarkers in health and disease.
To be effective, nanoparticles have to face the challenges of the body’s biological barriers. They have to avoid being removed from circulation too quickly
There are also challenges in manufacturing. As with all drugs in development, scaling up the quantities required from bench research, through clinical trial supplies to manufacturing for the market requires a lot of investment and expertise.
Other challenges that have to be met during manufacturing include:
● Stability during preparation, sterilisation and storage
● Efficiency in drug loading and encapsulation
● Particle size and size distribution
● Quality control and characterisation
● Regulatory considerations
Since the introduction of the concept of nanoparticles in 1959 by the American physicist and Nobel Prize laureate Richard Feynman, nanoparticles have taken a growing role across science. They are already playing an important role in drug delivery, and their uses will become more widespread as technology improves.
Based in the north of England, Suzanne Elvidge is a freelance medical writer with a 30-year experience in journalism, feature writing, publishing, communications and PR. She has written features and news for a range of publications, including BioPharma Dive, Pharmaceutical Journal, Nature Biotechnology, Nature BioPharma Dealmakers, Nature InsideView and other Nature publications, to name just a few. She has also written in-depth reports and ebooks on a range of industry and disease topics for FirstWord, PharmaSources, and FierceMarkets. Suzanne became a freelancer in 2006, and she writes about pharmaceuticals, consumer healthcare and medicine, and the healthcare, pharmaceutical and biotechnology industries, for industry, science, healthcare professional and patient audiences.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


